INTRODUCTION
Inhaled furosemide, one of the loop diuretics, has been shown to have a protective effect against bronchoconstriction in asthma induced by exercise 1, hyperventilation in cold air 2, inhalation of ultrasonically nebulized distilled water 3, and other stimuli 4. These studies have shown that when furosemide is administered as an aerosol, it can prevent or ameliorate asthma exacerbations. However, a therapeutic effect has not yet been established.
The purpose of this study was to investigate whether inhaled furosemide has a therapeutic effect in children with acute asthma.
PATIENTS AND METHODS
Patients
Children who were diagnosed with asthma according to the criteria of the American Thoracic Society 5 were included in the study.
The inclusion criteria were:
1. Mild or moderate asthma exacerbation with a clinical asthma score of between 1 and 6.
2. Age between 5 and 14 years.
3. Ability to perform a peak flow meter test.
Study design
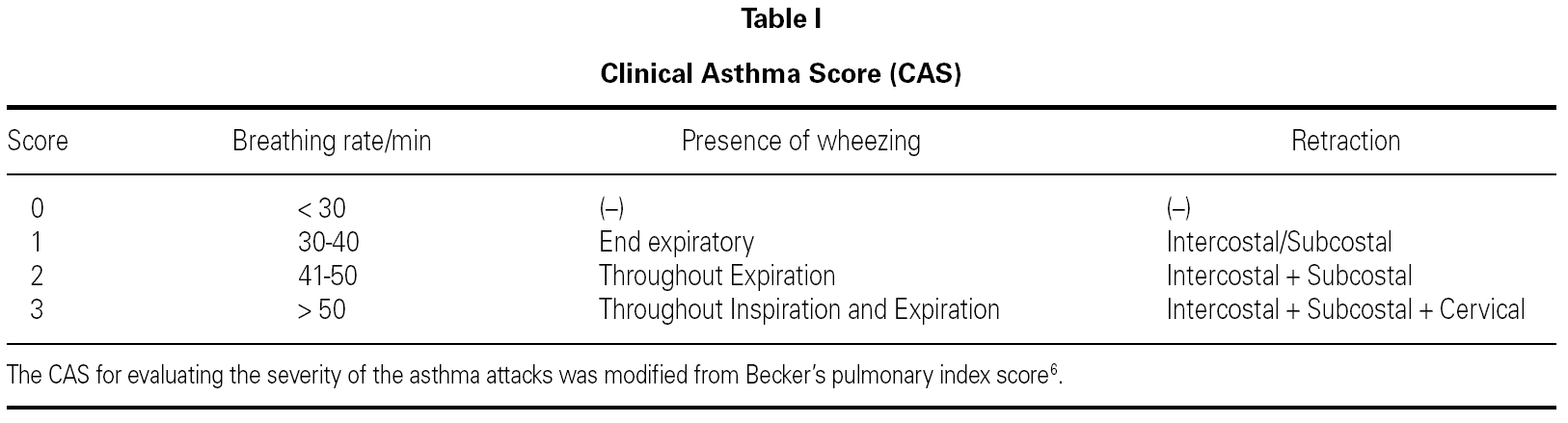
The study design was double-blind and placebo-controlled. Patients were randomized to receive either nebulized salbutamol (0.15 mg/kg) (Ventolin nebules 2.5 mg/2.5 mL, Glaxo Wellcome Inc.) plus nebulized furosemide (10 mg/m 2) (Lasix 20 mg/2 mL, Aventis Pharmaceuticals Inc.) (Group 1), or nebulized salbutamol (0.15 mg/kg) alone with nebulized saline as placebo (Group 2). Randomization was performed according to the patient's social security number. In all patients, the clinical asthma score (CAS) was determined before and after treatment. The CAS was modified from Becker's pulmonary index score 6 (table I).
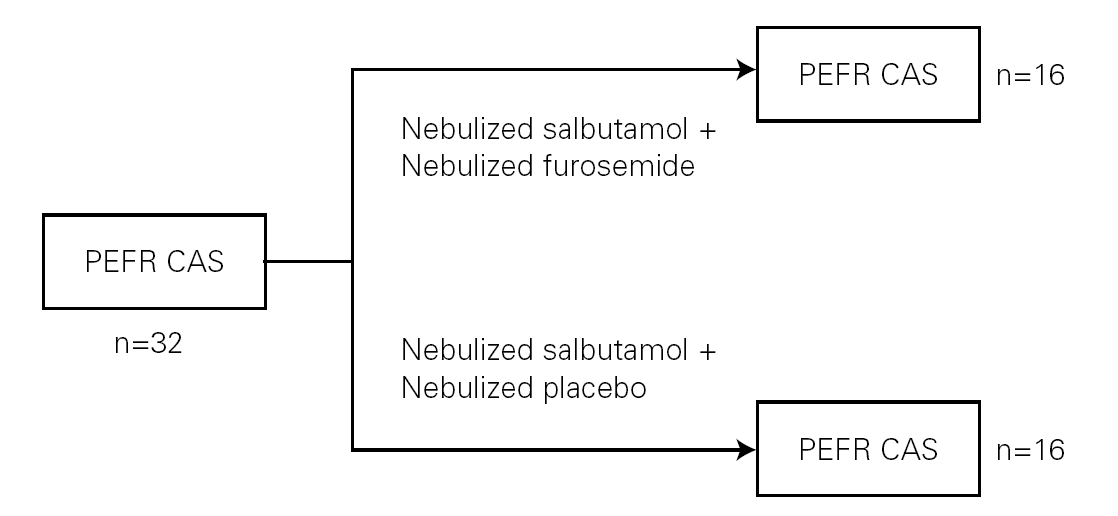
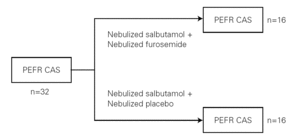
Peak expiratory flow rates (PEFR) were measured by a peak flow meter (Clement Clarke, Essex, England). The best of the successfully performed maneuvers was recorded (fig. 1).
Figure 1.--Study design. CAS: Clinical Asthma Score; PEFR: Peak Expiratory Flow Rate.
Statistics
Age was compared between the two groups with the Mann-Whitney U test. Gender was compared with Fisher's exact test. CAS and PEFR values before and after treatments were compared with the Mann-Whitney U test. Pre- and post-treatment CAS and PEFR values in the study and control groups were compared with Wilcoxon's matched-pairs signed-rank test. The changes in pre- and post-treatment CAS and PEFR values in the study and control groups were compared with the Mann-Whitney U test. A p-value of < 0.05 was considered statistically significant. The statistical analysis was performed by Graphpad Instat ver. 3.05 (Graphpad Software Inc. San Diego, CA, USA).
RESULTS
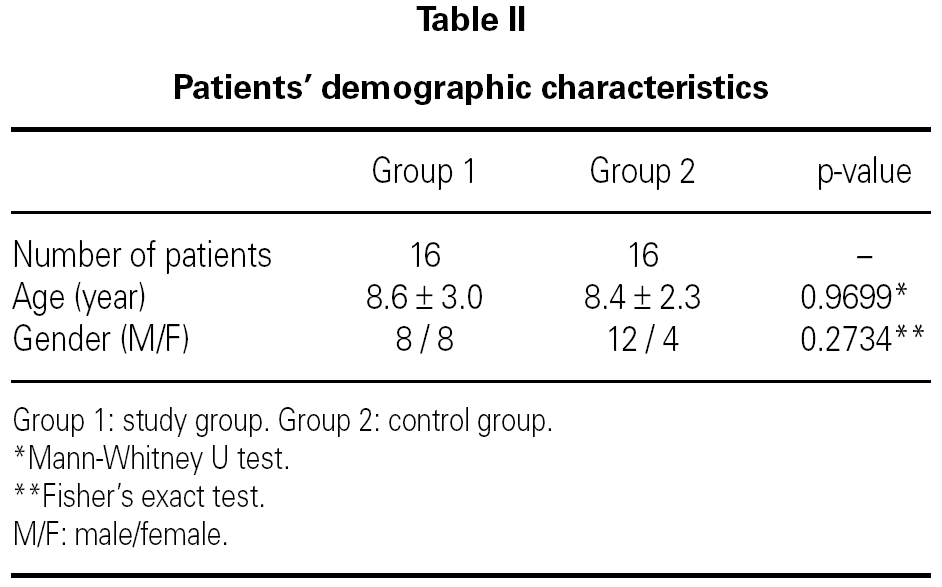
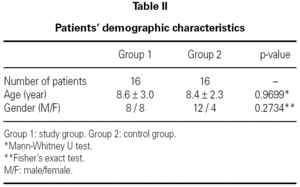
There were 16 patients in the study group (Group 1), with 8 boys and 8 girls. The mean age in this group was 8.6 ± 3.0 years (table II). There were 16 patients in the control group (Group 2), with 12 boys and 4 girls. The mean age in this group was 8.4 ± 2.3 years (table II). There was no statistically significant difference between the two groups with respect to gender or age (p = 0.2734 and p = 0.9699 respectively).
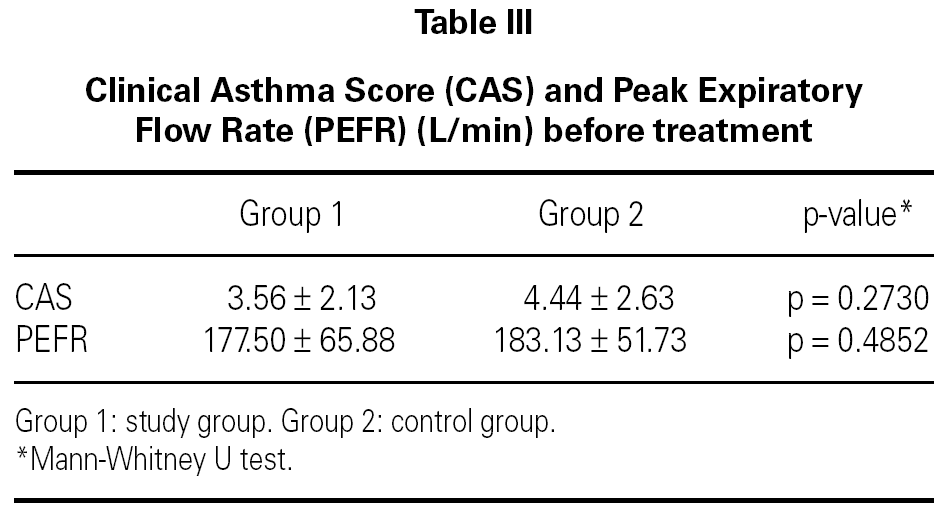
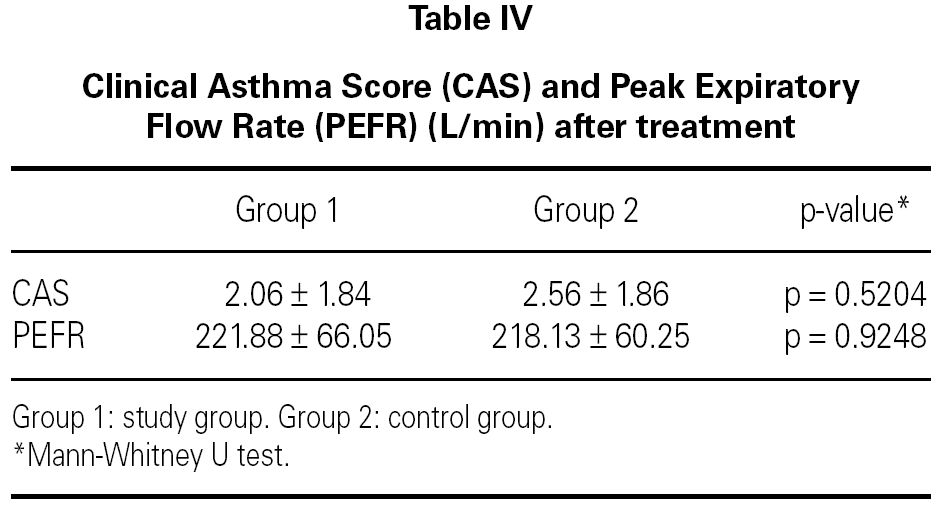
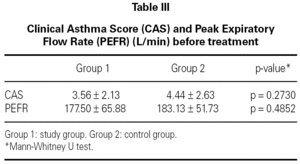
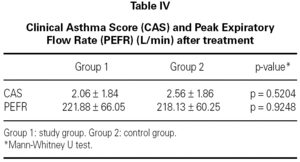
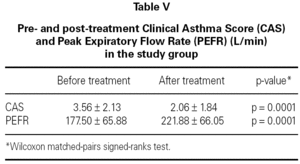
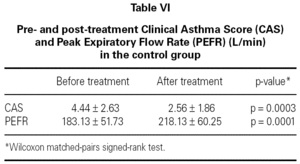
The mean baseline CAS was 3.56 ± 2.13 in the study group and 4.44 ± 2.63 in the control group. There was no statistically significant difference between the two groups with respect to baseline CAS (p = 0.2730) (table III). The baseline PEFR was 177.50 ± 65.88 in the study group and 183.13 ± 51.73 L/min in the control group. There was no statistically significant difference between the two groups with respect to PEFR (p = 0.4852) (table III). After treatment CAS was 2.06 ± 1.84 in the study group and 2.56 ± 1.86 in the control group. The difference was not statistically significant (p = 0.5204) (table IV). The post-treatment PEFR was 221.88 ± 66.05 in the study group and 218.13 ± 60.25 L/min in the control group. This difference was not statistically significant (p = 9248) (table IV).
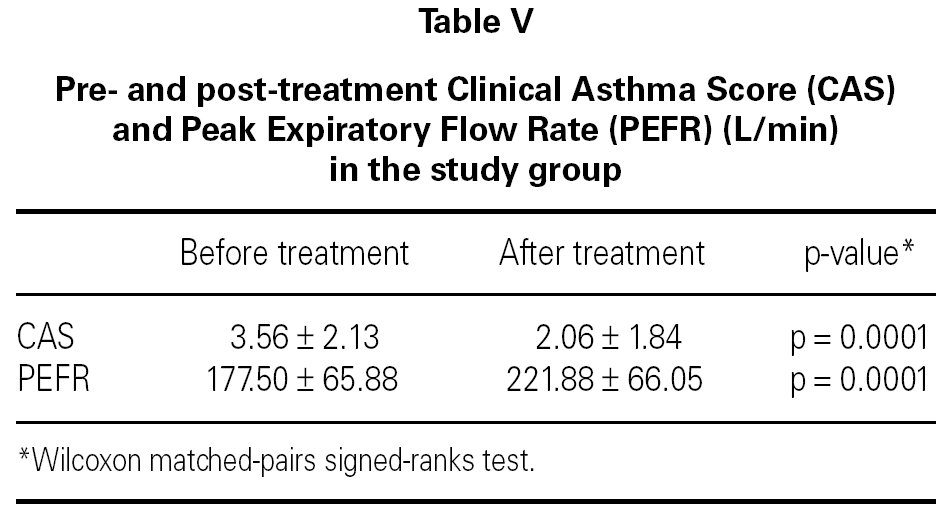
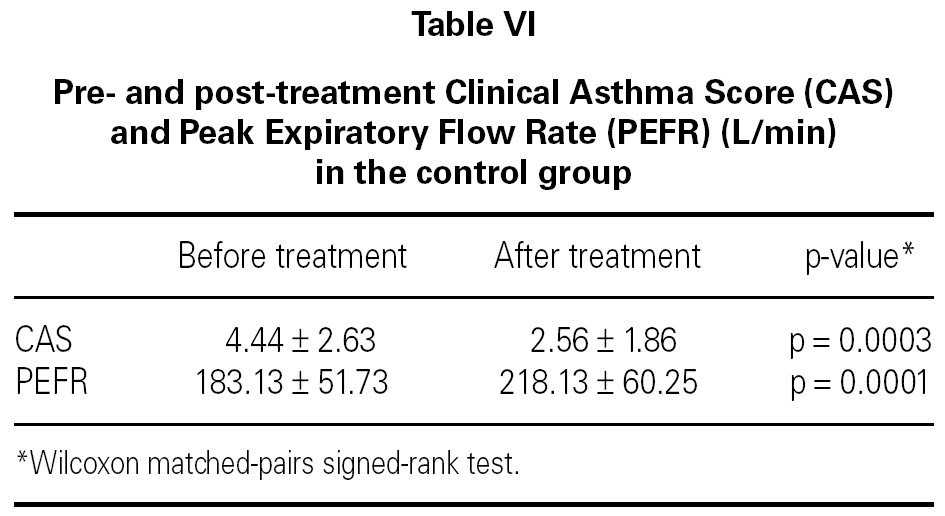
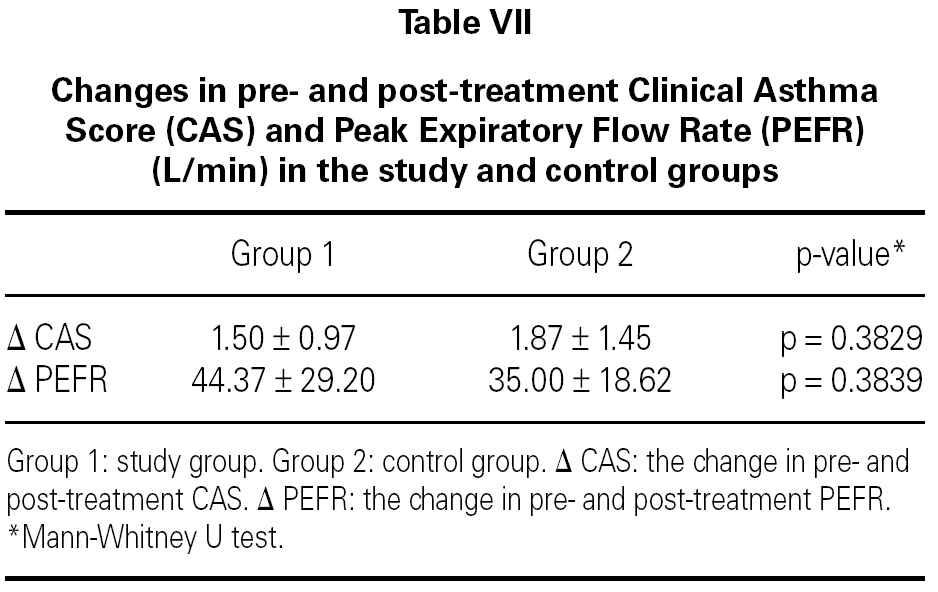
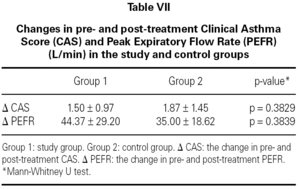
In both groups, CAS and PEFR significantly improved after treatment (p = 0.0001 and p = 0.0001 respectively for Group 1 and p = 0.0003 and p = 0.0001 respectively for Group 2) (tables V and VI). However, the addition of nebulized furosemide to nebulized salbutamol did not produce a significantly greater improvement in clinical or PEFR values than nebulized salbutamol alone (table VII).
DISCUSSION
Although inhaled furosemide has been shown to have a protective effect against many bronchoconstrictive agents and exercise 7-10, its effectiveness in the acute setting is still debated.
Our study compared inhaled salbutamol alone with a combination of inhaled salbutamol and inhaled furosemide in children with acute asthma. Our results demonstrate that the addition of inhaled furosemide to inhaled salbutamol did not improve clinical or PEFR parameters.
The first study to investigate the effectiveness of inhaled furosemide in the acute setting was conducted by Karpel et al 11. These investigators compared three groups: the first group received inhaled furosemide 40 mg, the second group received metaproterenol 15 mg and the third group received 40 mg furosemide plus 15 mg metaproterenol. Forced expiratory volume in 1 second (FEV1) was measured at 0, 15, 30, 45 and 60 min after the treatment. FEV1 significantly increased in the patients treated with metaproterenol alone but the change in FEV1 produced by the addition of furosemide was not statistically different compared with that produced by metaproterenol alone. The results of these authors are fairly similar to our own, except that their study was conducted in adults.
Tanigaki et al 12 investigated the effectiveness of inhaled furosemide in 7 adults with acute asthma who had not responded to conventional therapy with sympathomimetic agents, corticosteroids, and aminophylline. These authors reported that the addition of furosemide to this conventional therapy significantly improved PaCO2.
The above study differs from our study in several respects, which may explain the dissimilar results. Firstly, the study by Tanigaki et al was conducted in adults. Secondly, their study was not double-blind and placebo-controlled and the number of patients included was very small. Thirdly, the patients in their study had severe acute asthma refractory to conventional therapy whereas our study investigated pediatric patients with mild or moderate asthma who had not received any acute treatment.
Another study in adults, conducted by Ono et al 13, investigated the effect of furosemide in addition to aminophylline plus hydrocortisone using a randomized, double-blind, placebo-controlled design. These authors reported that the addition of furosemide resulted in a statistically significant improvement in FEV1 and PEFR. The difference between the results of this study and those of our own may be due to the combination used and the age of the patients.
Pendino et al 14 investigated the effectiveness of adding nebulized furosemide to nebulized salbutamol in adult patients referred to the emergency department with an acute asthma attack. The patients were evaluated both clinically and with PEFR. This double-blind, placebo-controlled study was very similar to our own with respect to the study design and results but differed in that our study was conducted in children. Pendino et al report that adding nebulized furosemide to nebulized salbutamol did not produce greater improvement than placebo. However, they report that a subgroup of patients that had been referred to the emergency department within the first 8 hours of the attack showed a significantly greater improvement in both variables. In our study we did not divide the patients according to the duration of the attack. This could be the subject of future investigations in which patients with short attacks are evaluated.
Another double-blind, placebo-controlled study with similar results to those of our own was conducted by Rodrigez et al 15. These investigators evaluated adult patients with acute asthma attacks treated by nebulized furosemide in addition to nebulized salbutamol and found no statistically significant differences in spirometric values.
Recently, Hinckley et al 16 reported no statistically significant differences in adult patients treated with nebulized albuterol plus nebulized furosemide in comparison with patients treated with nebulized albuterol plus placebo.
Gonzalez-Sanchez et al 17 conducted a double-blind, placebo-controlled pediatric study to investigate the effectiveness of the combination of nebulized albuterol plus nebulized furosemide compared with placebo and found no significant differences in spirometric values (FEV1). The study design and results of this study are fairly similar to those of our own.
We conclude that the addition of nebulized furosemide to nebulized salbutamol in pediatric patients with an acute asthma attack does not produce greater improvement in clinical and spirometric parameters than nebulized salbutamol alone. The effectiveness of this combination in the subacute phase of the attacks could be the subject of future analyses.
Correspondence:
Çag(breve)atay Nuhog(breve)lu, MD
Yes¸ilbahar sokak, Savas¸ Apt. No: 16/16
Göztepe. 34730 Istanbul. Turkey
E-mail: cnuhoglu@e-kolay.net
Web: http://www.haydarpasanumune.gov.tr