INTRODUCTION
Pine processionary caterpillar (Thaumetopoea pityocampa), belonging to the Thaumetopoeidae family, can induce skin eruptions, generally located in exposed areas, and less frequently ocular lesions. Usually these reactions are produced by a toxic-irritative mechanism 1-3 motivated by airborne urticating hairs of the caterpillar 1, which, upon entering and breaking inside the skin produce a basophil degranulation with histamine release 4,5.
This caterpillar represents a real pest in Europe, mainly in the Mediterranean area 1. The urticating capacity of its hairs is well known from antiquity, however the first descriptions were made by Reaumur in 1736 and by Fabre in 1900 3. Since then different studies have provided new advances on the etiopathogeny of these reactions, which involve mechanical and chemical factors 2,6,7. Although this is the main pathogenic mechanism, they are more and more frequent bibliographical references of cases in those a mechanism of immediate hypersensitivity is implied, generally due to an occupational exposition 1-3,8.
This study emphasizes the appearance of IgE-mediated symptomatology produces by this Lepidoptera in children more frequently than previously observed.
PATIENTS AND METHODS
Patients
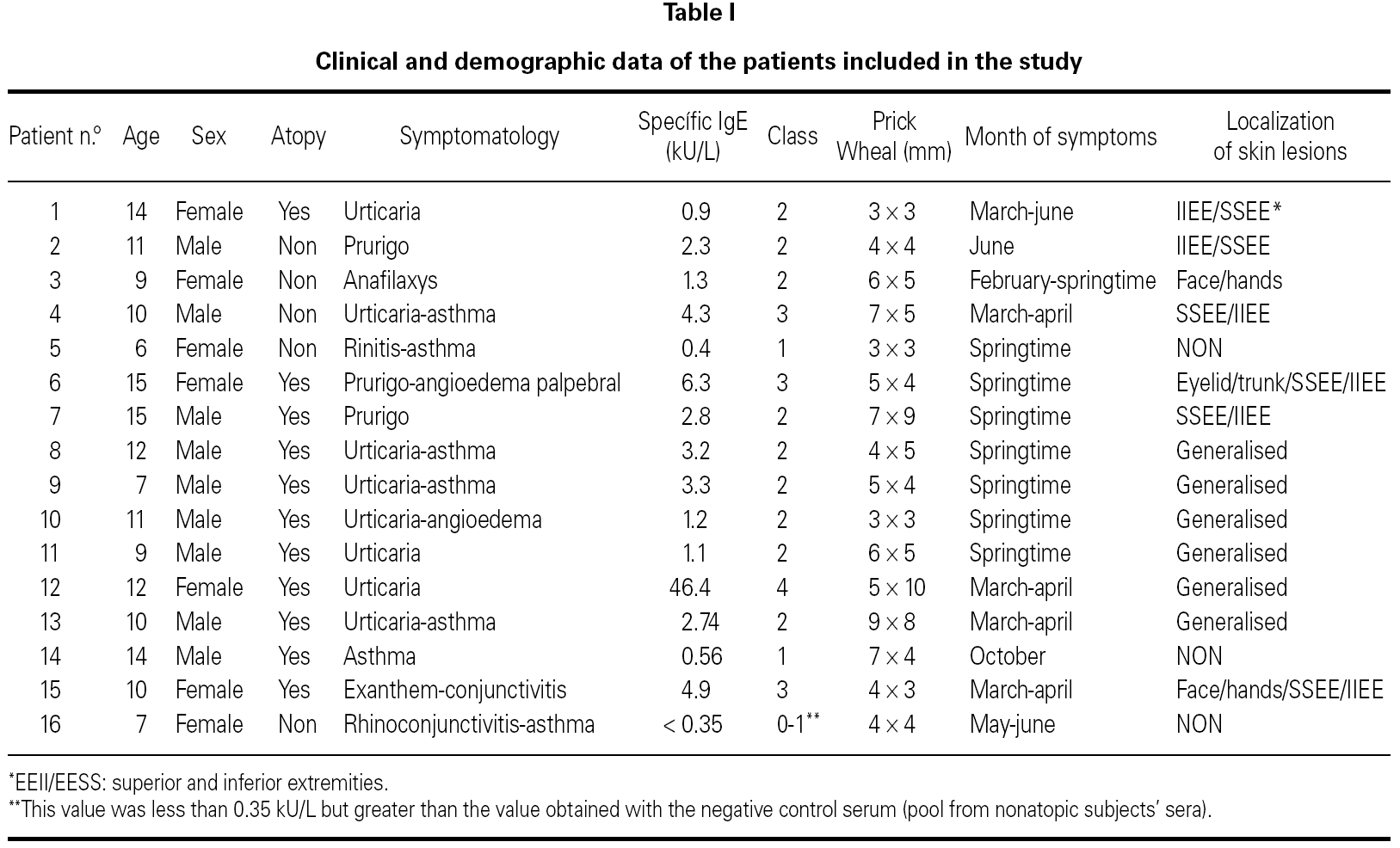
We evaluated sixteen children from 6 to 15 years old, who came to our clinical setting with different allergic symptoms probably related to pine processionary caterpillar exposure. Clinical and demographic data of patients are shown in table I.
Complementary clinical tests
All patients underwent a basic physical examination and a blood sample analyse which included hemogram with leukocyte formula, erythrocyte sedimentation rate (ESR) and biochemical blood analysis, as well as a fecal parasitologic examination when urticaria was the main clinical manifestation, and spirometry in presence of asthma.
Skin test
Skin prick tests (SPTs) were carried out with extracts of common aeroallergens (pollens, animal epithelias, moulds, mites and cockroaches), from Anisakis simplex and from caterpillars at the last larval stage (L5), provided by Laboratorios Bial-Arístegui. The caterpillar extract was also tested in 30 control subjects (atopic and non-atopic). Besides, SPT with mosquito (Aedes sp) extract was carried out in three patients with prurigo disease (patients n.º 2, 6 and 7) and in patient n.º 12.
Histamine phosphate (10 mg/ml) and sterile 0.9 % saline were used as positive and negative controls, respectively. A mean wheal area of 3 mm 2 or greater compared with the negative control, measured 15 minutes after puncture, was considered a positive response.
Preparation of pine processionary caterpillar extract
Some specimens of Thaumetopoea pityocampa in L5 larvae stage were ground in a pool of liquid nitrogen into a course "powder" of frozen fragments in a mortar and extracted by magnetic stirring in agitation in 50 mM phosphate-buffered saline (PBS) at pH 7.5 during 4 h at room temperature. After centrifugation, supernatant was dialyzed against water. The dialyzed extract was filtered through a 0.22 μm-pore diameter membrane and freeze-dried.
Determination of specific IgE
The level of serum specific IgE to common aeroallergens and Anisakis simplex was measured by CAP (Pharmacia Diagnostics, Uppsala, Sweden). Measurement of specific IgE to pine processionary caterpillar was performed by EAST method using Bial-Aristegui discs with the allergen coupled (10 mg/ml). Cellulose discs were activated with BrCN following the method of Ceska et al 9 and measure was carried out with the HY-TEC EIA Kit for specific IgE (HYCOR Biomedical Ltd. UK) following the manufacturer instructions.
SDS-PAGE Immunoblotting
SDS-PAGE was carried out according to the method of Laemmli 10, 12.5 % and 4 % of acrylamide were used for separating and stacking gel respectively. Samples were studied in two conditions: reduced (with β-mercaptoethanol) and non-reduced conditions (without β-mercaptoethanol). Separated proteins bands were electrophoretically transferred to polyvinylene difluoride (PVDF) essentially described by Towbin et al 11 and after incubation with patients' sera detection was performed by a chemiluminescence method as recommended by the manufacturer (ECL-Plus; Amersham Pharmacia Biotech).
RESULTS
Complementary clinical tests
Physical examination and complementary analyses performed were normal.
Skin test
Skin tests against pine processionary caterpillar extract were positive in all patients and negative in controls.
SPT against common aeroallergens was positive to pollens in 68.75 % of patients, to mites in 6.25 %, to moulds in 6.25 % and to epithelia in 25 %.
SPT to mosquito extract was negative in patients n.º 2, 6 and 7, and positive in patient n.º 12 who suffers from allergy to shellfish and showed skin sensitization to Anisakis simplex. His positive reactivity could be related to a crossed reactivity. Also, positive SPT to A. simplex extract was also observed in patient n.º 6.
Measurement of specific IgE
Results of specific IgE determination are shown in table I. Serum specific IgE against pine processionary caterpillar was positive in all cases, except in patient n.º 16. Significant high levels of specific IgE (class = 2) were detected in 81 % of the sera (13/16).
Immunoblotting
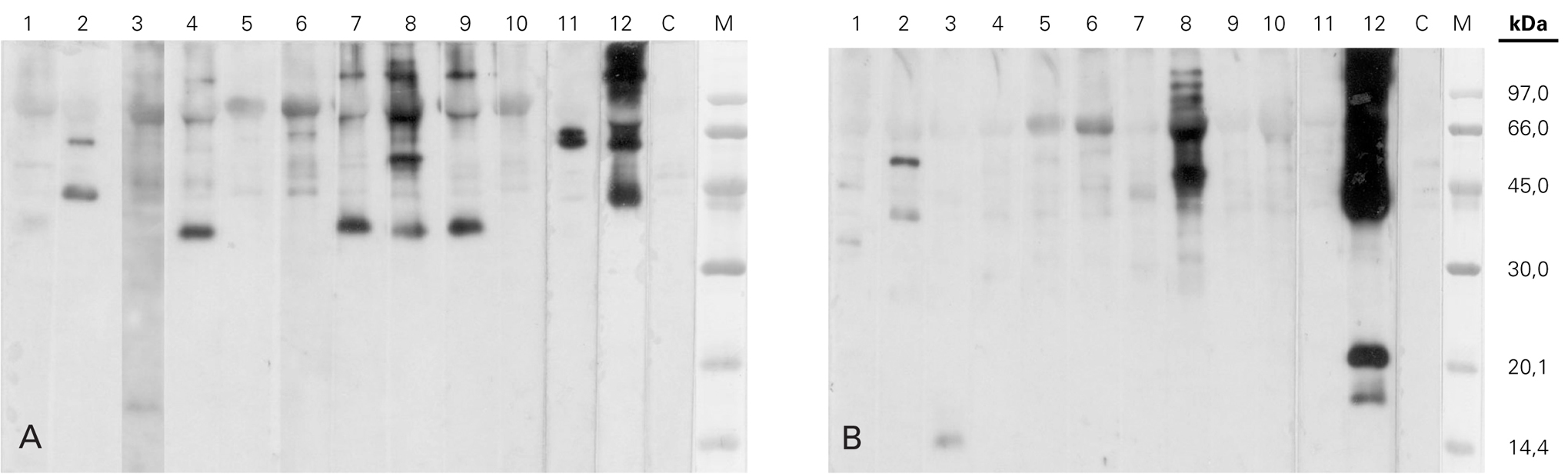
Results of SDS-PAGE Immunoblotting with caterpillar extract were different depending on the conditions in which the sample was prepared: in non-reduced condition (without β-mercaptoethanol), IgE-binding bands of 168, 70, 60, 64, 57, 44, 37 and 17.5 kDa were detected whereas in reduced ones (with β-mercaptoethanol) the molecular mass of the bands were 70, 55, 50, 40, 33, 21, 18 and 17/15 kDa (fig. 1). It is highlighted the presence of a band of approximately 37 kDa which was only detected in absence of β-mercaptoethanol, in with 80 % of the studied patients' sera (lanes 1, 4, 7, 8 and 9). This fact suggest the need of disulfide bridges to keep the native structure of this protein.
Figure 1.--SDS-PAGE Immunoblotting of the pine processionary extract incubated with the patients' serum . A) Sample without β-mercaptoetanol (non-reducing conditions). B) Sample with β-mercaptoetanol (reducing conditions). Lane 1: Patient 1 serum. Lane 2: Patient 2 serum. Lane 3: Patient 3 serum. Lane 4: Patient 4 serum. Lane 5: Patient 5 serum. Lane 6: Patient 6 serum. Lane 7: Patient 7 serum. Lane 8: Patient 8 serum. Lane 9: Patient 9 serum. Lane 10: Patient 10 serum. Lane 11: Patient 11 serum. Lane 12: Patient 12 serum. Lane C: Control serum (pool from nonatopic subjects' serum) M: Molecular mass marker.
DISCUSSION
Pine processionary is one of the main European forest pests 1,12. Approximately 150 species of lepidoptera have been described which are able to be harmful on human skin 2,3. Several species of caterpillars in larvae phase are equipped with an urticating mechanism provided with chitinous spines able to penetrate the dermis and to induce contact dermatitis 4. The effects of these urticating hairs in people are mainly skin injuries (dermatitis and contact urticaria), conjunctivitis, and, occasionally, bronchial effects and anaphylactic shock 1,2,13-15. These spicules are detectable in air by aerobiological methods and because of its size, (approximate length of 150-200 μm and diameter of 5 μm), its may penetrate in the human respiratory systems as far as the trachea and zones of the primary bronchi, inducing respiratory pathology 6,16.
Over the last years and generally in adult population, several studies reported 1,2,8,13,17,18 the existence of a hypersensitivity by an IgE-mediated mechanisms in a high percentage of the reactions to this caterpillar.
Lamy et al in 1986 4, and Werno et al in 1993 8 described an specific IgE-binding band of 28 kDa by western blot composed by two polypeptides of 13 and 15 kDa 4, and they identified it as Thaumetopoein. More recently, Moneo et al 19 described an IgE-binding protein of 15 kDa as the major allergen of pine processionary (Tha p 1). This latter author underlined that this protein showed the same molecular mass regardless of the electrophoretic conditions (reduced or non-reduced) and demonstrated the monomeric nature of the Tha p 1 protein.
In our study, Immunoblotting in absence of β-mercaptoethanol showed an IgE-binding band of 37 kDa in 80 % of the patients sera. In a previous study we detected an IgE-binding band by western blot of similar molecular mass with two of the patient sera (patient 2 and 4) 17. These results indicate the importance of carry out immunoblotting studies in presence and absence of β-mercaptoethanol: if the assay is only performed in standard conditions (with β-mercaptoethanol), the presence of certain relevant allergens as the 37 kDa one here indicated, could be not detected. In reduced conditions, serum of patient n.º 12 revealed a pair of IgE-binding bands of approximately 21 kDa/18 kDa, similar to those appeared with the sera of the two patients reported in our previous series 17. With the patient's serum 3, under reduced conditions, the fixation of IgE is detected in proteins of 15 and 17 kDa whose molecular mass would coincide initially with the most relevant alergen found in a study performed with sera from 16 patients who suffer from contact urticaria 18 described by Vega et al. This allergen was latter also described by Moneo et al when they characterized Tha p 1 as the mayor allergen from Thaumetopoea pityocampa 19.
In geographical areas with plenty of pine trees, outpatient pediatric consultations for symptoms related to pine processionary are frequent. Nevertheless, studies carried out in children are scarce 15,20 and epidemiological studies have not been underwent.
As far as we know only in three previous studies an IgE-mediated mechanism has been pointed out as the cause of hypersensitivity to this caterpillar during the childhood 15,17,20. One of them, which included 653 patients aged from 3 to 17 years, demonstrated that reactions to pine processionary affects 9.2 % of children and teenagers who frequent pine areas. Our study results agree with Vega et al findings 1,15,20, demonstrating that the most common clinical manifestations were the dermatological ones, with lesions generally located in exposed areas 1. This latter author also described some severe symptomatology like asthma and anaphylactic reactions, in patients exposed to high levels of allergen due to occupationally exposure 2,13. In our study, in despite the absence of occupationally exposure, six patients showed associated respiratory pathology; one of them reported asthma as the only clinical manifestation symptoms, and another one suffered an anaphylactic reaction.
In all of the cases here described symptoms appeared mainly between February and April (larvae phase L5), period of the year with the highest presence of caterpillar hairs in the air. Symptoms always appeared several hours after patients have been in pines areas infected with pine processionary caterpillar. Only in one case (patient n.º 14) symptomatology occurs in autumn, period of the year when pine processionary is in larvae phase L3-L4, an urticating but not procession state, and period when anaphylactic reactions are more frequent 2. Vega et al 2 noted that 37 % of the occupationally exposed patients present symptomatology from October to December, whereas in non-occupationally exposed patients symptoms generally appeared in springtime. Finally, the atopic status of the patients here studied is in accordance with the high percentage of atopic patients found by other authors among non-occupationally patients 2.
Therefore, the airborn urticating hairs of T. pityocampa should be considered, also in children, as seasonal inhalant allergens. In areas where the presence of this caterpillar is endemic, reactions to pine processionary caterpillar proteins should be taken into account in the diagnosis of urticaria, dermatitis and other allergic pathologies in children.
Correspondence:
V. Fuentes Aparicio, MD
Pl. Reyes Magos, 9, 6.ºB
28007 Madrid. Spain
E-mail: vfuentesaparicio@hotmail.com