INTRODUCTION
Specific immunotherapy (SIT) is the only treatment that interferes with the basic pathophysiological mechanisms of allergic disease1 and is widely used in the management of clinically-significant IgE-mediated respiratory allergic diseases. SIT has been used since 19112 proving efficacious both in allergic rhinoconjunctivitis and bronchial asthma3,4. As SIT is able to modify the immune response in early stages, it may also be effective in the prevention of new allergic sensitisations. Prior to the present study, only one group of researchers had addressed this topic, drawing different conclusions depending on whether patients were mono or polysensitised5,6 being both referred to paediatric populations. Recently, it has been published another paediatric study showing a lower development of new sensitizations in the SIT group compared to the control group7. Moreover, the European multi-center Preventive Allergy Treatment study show that after 3-year SIT and 5-year follow-up, the risk for onset of asthma is reduced in children 5 to 13 years old suffering from allergic rhinoconjunctivitis, but no results on development of new sensitizations are available8,9.
The aim of the present study was to assess the ability of SIT to prevent the development of new sensitisations in monosensitised patients. The influence of other factors (age, sex, atopy background, family pets and smoking) on the development of new sensitisations was analysed and the clinical outcome compared in patients treated with or without SIT.
PATIENTS AND METHODS
Patient population
The first 100 consecutive patients attending for the first time an allergy outpatient clinic of a general hospital after 1990 and meeting the following inclusion criteria were selected:
1. Diagnosis of allergic rhinitis and/or asthma established after a conventional work-up study and evaluated by symptom score. Rhinitis was expressed as absent, mild, moderate or severe, and asthma was evaluated by the four degrees of the Global Initiative for Asthma10.
2. Monosensitisation to one of the three more prevalent allergens in the area (Dermatophagoides spp, Parietaria judaica pollen and grass pollen).
3. Complete follow-up in the same allergy outpatient clinic for 3 to 5 years.
The selected patients were offered a comprehensive interview covering recent data on family atopy history (first-degree relatives), smoking habits, having pets at home (cat and/or dog) and the same symptom score used for the initial diagnosis by a blinded investigator who also performed prick tests with the same aeroallergen battery as that used at the time of the initial diagnosis. The patients were divided in a SIT-treated group (group A) and a group treated with conventional drugs and environmental measures alone (group B).
Skin prick test
The skin prick tests were performed with biologically-standardised extracts, if possible, and of the same commercial brand. The extracts included house dust mites (Dermatophagoides farinae, Dermatophagoides pteronyssinus), cat and dog dander, main pollens in the area (Cupressus arizonica, Corylus avellana, Platanus acerifolia, Olea europea, Parietaria judaica, Artemisia vulgaris, Plantago lanceolata, Mercurialis annua, Salsola kali, Phleum pratense, Cynodon dactylon and Phragmites communis), common moulds (Alternaria alternata, Cladosporium herbarum, Aspergillus spp, and Penicillium spp) and latex. Positive (histamine chloride 10 mg/ml) and negative (saline) controls were used. Skin prick tests were performed in the same order and on the same volar surface of the forearm in both evaluations. A mean diameter greater than 3 mm was considered positive if no dermographism and/or positivity of negative control were recorded.
Immunotherapy
SIT was performed using commercial extracts of biologically-standardised extracts of Dermatophagoides farinae or Dermatophagoides pteronyssinus, (whatever the greater skin test area), Parietaria judaica and grass mix pollen, according to the usual protocol in the unit and consisted of an initiation phase of gradually increasing doses in which the injections were administered weekly, and a maintenance phase with the standard commercial dose (or the highest dose tolerated by the patient) was administered at 4-week intervals until at least 3 years of treatment had been completed.
Statistics
Descriptive analysis was performed using absolute and relative frequencies for categorical variables and medians and ranges for quantitative variables. The occurrence of new sensitisations in SIT-treated and untreated patients was compared with the chi-square test. A parametric test (t-test) was used for quantitative variables, calculated as relative risk (RR) with 95 % confidence interval (95 % CI). P values ≤ 0.05 were considered statistically significant. All analyses were performed with the SPSS 6.1 statistical software package.
RESULTS
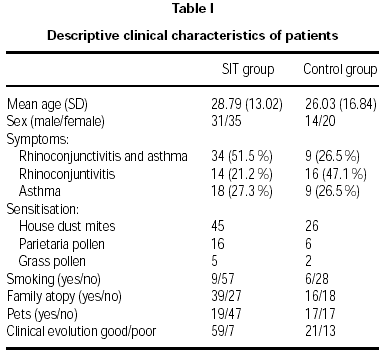
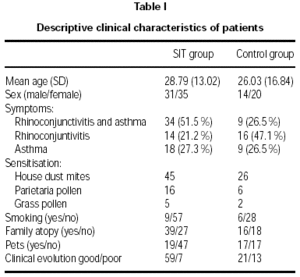
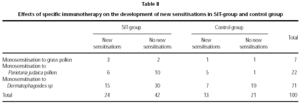
Forty-five males and fifty-five females aged 6 to 69 were recruited. Seventy-one were monosensitised at baseline to Dermatophagoides, twenty-two to Parietaria judaica pollen and seven to grass pollen. Of the 100 patients, 66 were treated with SIT (group A) and 34 with avoidance of allergen and conventional drugs alone (whatever the inhaled corticosteroids, bronchodilators, local or oral antihistamines or nasal corticosteroids).
Age and sex of both groups were comparable at baseline. Prevalence of asthma was higher in the SIT-treated than in the non-SIT-treated group (table I).
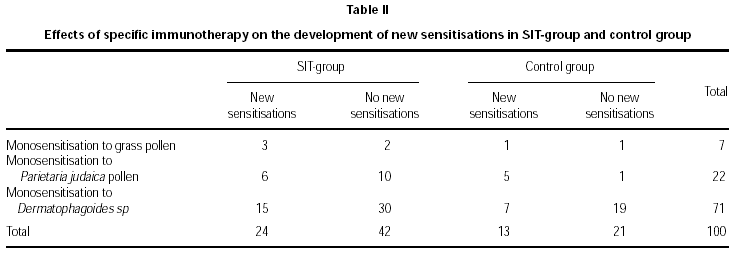
No statistically-significant differences were observed between the groups in the rate of new sensitisations (RR = 0.97, CI 95 %: 0.72-1.3), since 36.4 % of SIT-treated patients showed new sensitisations, compared with 38.2 % of the control group (table II). Smoking, family atopy history and pets did not appear to be a risk for developing new sensitisations (p < 0.05). Nevertheless, the SIT-treated group showed better clinical outcome compared to the control group, with an improvement of 89.4 % versus 61.8 % (p = 0.007).
DISCUSSION
The present study, which evaluated 100 consecutive monosensitised patients aged between 6 and 69, showed no differences in the development of new sensitisations between patients treated by 3 to 5-year SIT and patients not treated with SIT. Prior to the start of the study, only two published reports had analysed the effect of SIT on the rate of new sensitisations and both referred to paediatric populations. One included polysensitised patients and concluded that SIT did not prevent the development of new sensitisations5. The other, a prospective case-control of 44 asthmatic children aged 2 to 6 years, showed that all children in the control group developed new sensitisations, versus only 12/22 of the 3-year SIT-treated group6. Preliminary data of another paediatric study, the ongoing European multi-centre Preventive Allergy Treatment (PAT)8,9, clearly show that after 3-year SIT and 5-year follow-up, the risk for onset of asthma is reduced in children 5 to 13 years old suffering from allergic rhinoconjunctivitis, but no results on development of new sensitisations are available to date.
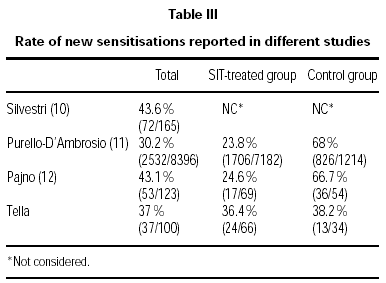
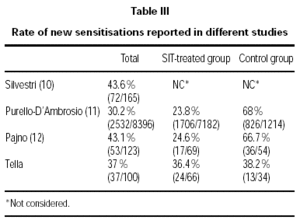
Other articles on development of new sensitisations have recently been published7,11,12. In the two studies conducted in the south of Italy, the development of new sensitisations was found to be statistically lower in the SIT group compared to the control group. However, when the total number of patients in each study is considered, the rate of new sensitisations ranges from 30 to 43 % with the highest percentages being in paediatric series (table III). These studies were planned as retrospective and observational and did not define medical judgement to prescribe SIT, thereby slanting the results obtained. Therefore, new randomised studies are required to draw definitive conclusions regarding the effect of SIT on preventing new sensitisations in monosensitised patients.
A family history of atopy did not influence the development of new sensitisations in our patients, probably due to the selection of monosensitised patients, since a background of atopy is usually more prevalent in polysensitised patients. In our study, neither smoking nor pet exposure influenced the development of new sensitisations. In this respect, recent information casks some doubt on the role of smoking and exposure to pets in the development of allergic sensitisation13-21.
From a clinical point of view, the degree of reduction in symptoms and/or drug intake was significant when the SIT-treated group and non-treated group were compared, thereby reflecting the efficacy of SIT.