INTRODUCTION
Common variable immunodeficiency (CVID) is the most clinically relevant primary antibody deficiency in the adult age 1-2. Hypogammaglobulinemia and defective antibody production are the hallmark of CVID syndrome, predisposing patients to recurrent bacterial infections. An increased frequency of lymphoproliferative disease, splenomegaly, granulomatosis and or autoimmune diseases is also observed in CVID patients 1-3. Many reports describe peripheral blood T and B lymphocyte dysfunctions in a substantial proportion of CVID patients 4-5. Recent studies point toward defects at various stages of B-cell differentiation in CVID subgroups and support the value of a B-cell-oriented classification principle 6-12. A good correspondence between a low number of memory B cells and splenomegaly, lymphoid proliferation, granulomatous disease and/or autoimmune diseases has been suggested 6-12. In this study, we investigated if the alterations in peripheral T-cell activated lymphocyte subsets correlated with clinical findings in CVID patients.
MATERIAL AND METHODS
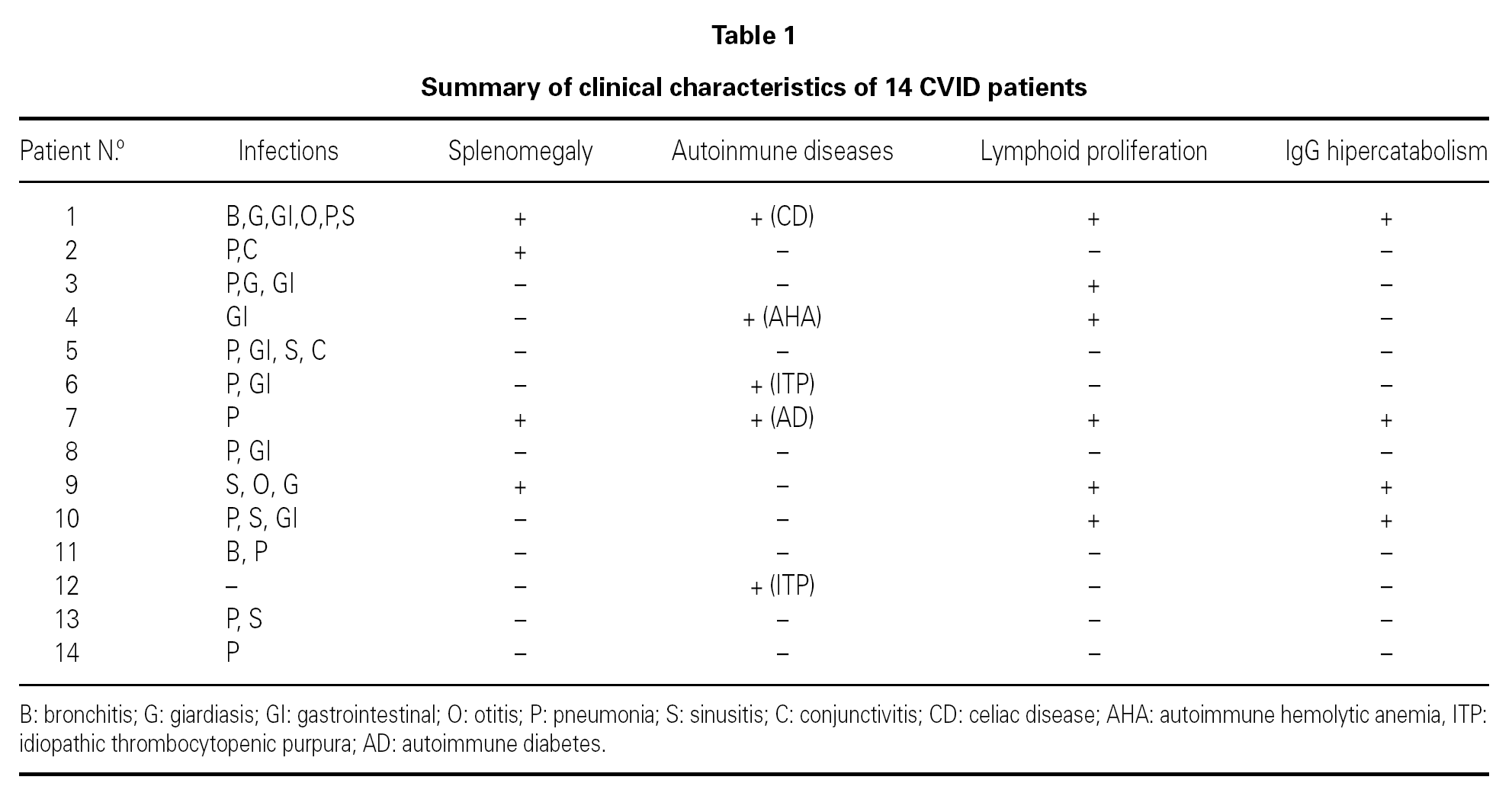
In a transversal descriptive study we analyzed activated CD4+ and CD8+ T cell-subset percentages in 14 CVID patients (21-68 years old, mean age 37.4 years, 8 men and 6 women). All patients had been diagnosed as having CVID based on diagnostic criteria for primary immunodeficiencies 13. The clinical characteristics of the patients are summarized in table I. At the time of the immunological study all the patients were receiving substitutive therapy with intravenous immunoglobulin (IVIG), at a dose of 400 mg/kg every 3 weeks. Blood samples were obtained immediately before IVIG administration. Increased levels of lymphocyte activation markers can be observed during chronic infections. For that reason, in this study we compared the percentages of lymphocyte activated subsets in CVID patients with the percentages observed in 47 asymptomatic HIV-positive patients whithout clinical criteria of AIDS; and with 6 non-CVID hypogammaglobulinemic patients who had chronic bacterial infections and who were receiving IVIG. We also compared lymphocyte subsets percentages in CVID patients against values observed in a health control group composed of 23 healthcare workers. Splenomegaly and lymphoid proliferation were confirmed by computed tomography. Increased catabolism of serum IgG antibodies was suspected in those patients that did not reached IgG levels greater than 600 mg/dl despite conventional dosis of IVIG. None of the patients had protein losing diseases. Patients with acute infections or opportunistic infections were not included in the study.
Activated CD4 and CD8 T-cell subsets were quantitated by three color flow cytometry (FACScan, Becton & Dickinson, San Jose, CA, USA). We enumerated T-cell subsets using FITC/PE/PerCP combinations of CD38/HLA-DR/CD4 or CD8 and isotype controls. Percentages of activated CD4 and CD8 T cell subsets are expressed as a percentage of total CD4 and CD8 lymphocytes. Details of adquisition and analysis of lymphocyte subsets have been reported elsewere 14. T-cell activated percentages were compared between groups using the non-parametric Mann-Whitney test.
RESULTS
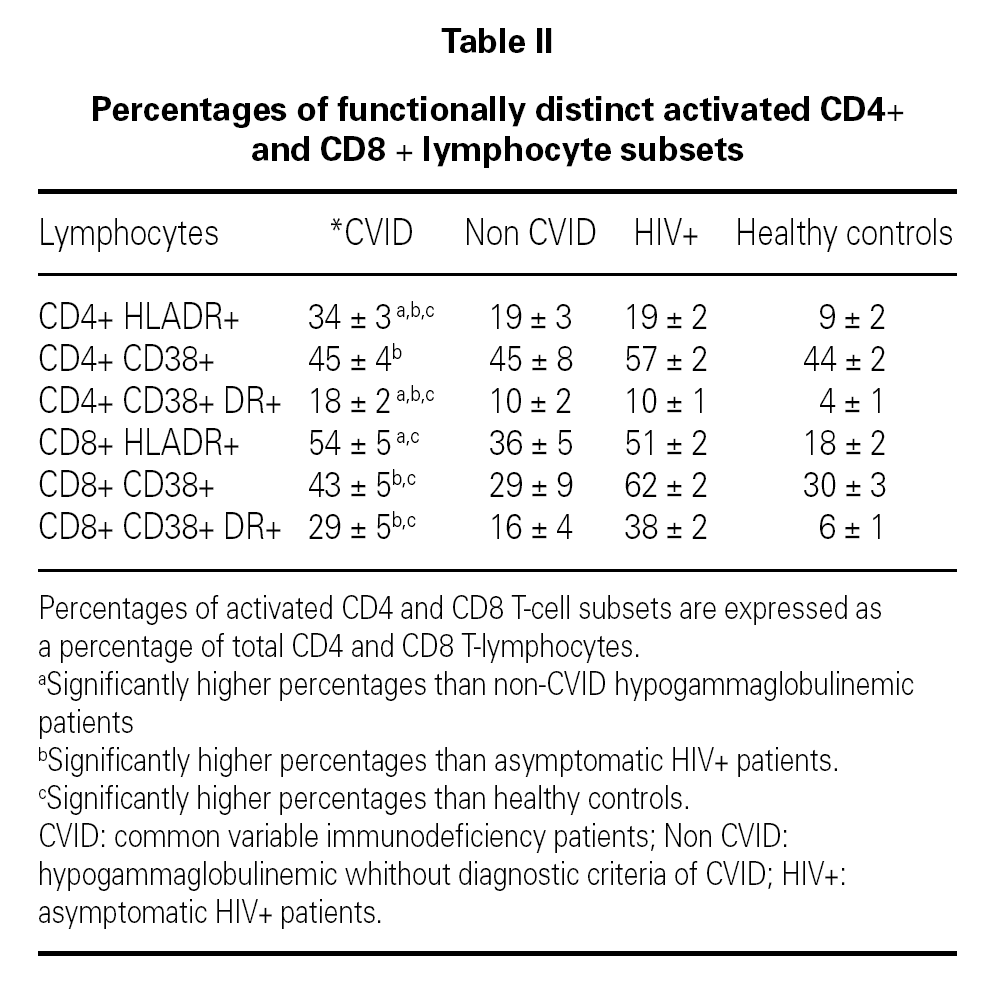
Mean concentration of serum IgG at the time of diagnosis of CVID was 127 mg/dl (15 to 441 mg/dl) and at the time of the immunological study it was 837 mg/dl (384 to 1320 mg/dl). Table II shows the mean percentages of the functionally distinct activated CD4 and CD8 T-cell subsets in the study groups. In CVID patients serum levels of CD4+ T-cells co-expressing the activation marker HLA-DR [CD4+ DR+ (34 %), CD4+ CD38+ DR+ (18 %)] were significantly elevated compared with all control groups. A tendency toward a negative correlation between the percentage of CD4+ CD38+ DR+ T-cells and the concentration of serum IgG at the time of the diagnosis of CVID was observed (Spearman Coefficient, r = 0.48 (p = 0.08)]. CVID patients showed higher percentages of CD8 + DR + T-cells (54 %) than HIV-negative controls. Significant increases of CD8+ CD38+ (43 %) and CD8+ CD38+ DR+ (29 %) T-cells were observed in CVID patients as compared with healthy controls. CVID patients with splenomegaly, lower pre-infusion IgG levels (< 600 mg/dl), autoimmune or lymphoproliferative conditions demonstrated even higher levels of CD4 + CD38 + DR + T cells (22, 22, 21 and 21 % respectively) compared with other CVID patients (13, 13, 15 and 15 % respectively).
Interestingly enough, the group of CVID patients whithout any complication (n = 5) showed higher percentages of CD4 + DR + (27 %), CD4+ CD38+ DR+ (12 %), CD8+ DR+ (49 %) and CD8+ CD38+ DR+ (22 %) T-cells than healthy controls.
DISCUSION
Recent studies have proposed a new classification of CVID patients using class-switched memory B-cell counts determined by flow cytometry in peripheral blood 8-9,11. CD27+ (memory) B lymphocytes can be divided into two distinct subpopulations: IgD-CD27+, which produces IgG, IgM or IgA (class-switched memory phenotype), and IgD+ CD27+, which predominantly produces IgM (non-class-switched memory phenotype). A good correspondence has been observed between a low number of memory B cells and splenomegaly, lymphoid proliferation and/or autoimmune diseases which are common complications in CVID patients 1-2,8-9,11.
Several T-cell abnormalities have also been observed in CVID patients including decreased lymphocyte proliferation in response to mitogens and antigens, a tendency towards increased T-cell apoptosis and elevated levels of T-cell activation markers 12,15-20. However, few studies have correlated T-cell functional abnormalities with clinical findings 12,16,20. Studies performed in acquired states of immunodeficiency, such as in HIV infection, have demonstrated that the increase in the percentages of CD4 and CD8 T-cell activated subsets provided additional independent prognostic information on the progression of the disease 14. We hypothezised that increased levels of CD4 and CD8 T-cells might be associated with clinical findings commonly observed in CVID.
Previous studies have compared the values of the functionally distinct subsets of T-cells observed in CVID patients with healthy controls. Since it is not easy to completely exclude the presence of infections in CVID patients, in this study we have compared the percentages of CD4 and CD8 T-cell activated subsets in CVID patients with the percentages observed in patients with a viral chronic infection (HIV + asymptomatic patients) or with chronic bacterial infections (non-CVID hypogammaglobulinemic patients with bacterial infections). Interestingly, CVID patients disclosed significantly higher percentages of activated CD4 T-cells than controls with chronic infections, which suggest that CD4 lymphocyte activation does not merely reflect a chronic infection state in CVID patients. Furthermore, when we split our patients into two groups according to the presence or absence of complications (splenomegaly, autoimmune disease and/or lymphoid proliferation), we found higher percentages of CD4+ CD38+ DR+ T-cells in CVID patients with such complications. In addition to previous studies, we have observed significantly higher percentages of CD4+ CD38+ DR+ T-cells in patients with suspected IgG hypercatabolism.
CVID patients showed increased percentages of activated CD8 T-cell subsets than healthy controls. It has been recently reported that the expresión of the HLA-DR molecule on CD8 T-cells was restricted to patients with clinical complications 20. In our study we observed higher percentages of CD8+ DR+ in CVID patients with clinical complications (splenomegaly, autoimmune disease and/or lymphoid proliferation), but the difference was not significant (data not shown). Since the percentage of CD8+ DR+ T-cells in CVID patients was similar to that observed in HIV positive controls, we speculate that a subclinical or undetected infection could at least partially explain the higher percentage of activated CD8+ T cells observed.
The results presented in this study indicate a state of ongoing T lymphocyte activation which is associated with clinical findings frequently observed in CVID patients. Further studies with a greater number of patients are necessary to establish if the evaluation of CD4 and CD8 T-cell activated subsets might be useful to identify subgroups of patients for etiological studies and classification.
ACKNOWLEDGEMENTS
The authors would like to thank Dr. Jose Miguel Benito for performing lymphocyte subset analysis of HIV + and healthy controls. They also acknowledge Dr. Juana Gil (Cellular Immunity Section) and Dr Margarita Rodriguez-Mahou (Autoimmunity Section) for laboratory diagnostic evaluation of the patients.
Correspondencia:
J. Carbone
Clinical Immunology Unit.
Immunology Department
Hospital General Universitario Gregorio Marañón
Dr. Esquerdo, 46
28007 Madrid. Spain
E-mail: carbone@teleline.es