An increase in asthma prevalence is reported from developed as well as developing nations, with rising costs from acute asthma and great expenditures to health care systems. Venezuela's Ministry of Health ambulatory facilities care for 80% or more of a mostly urban and impoverished population of 26 million inhabitants, registering close to a million acute asthma visits per year; a nebulised fixed fenoterol-ipratropium bromide combination (Berodual®, Boehringer-Ingelheim) in repeated dosing is the standard treatment.
Objectivesto simplify acute asthma care and management in a cost effective manner employing Formoterol Fumarate powder, a long acting beta agonist with immediate bronchodilator effects.
MethodologyFifty acute asthmatic children (5-12 years old) were randomly assigned (25 patients in each group) to receive either a nebulised single dose (US $1.35) of two 12 μg Formoterol Fumarate capsules (Foradil® 12 μg/cap, Novartis Pharma AG, Basel, Switzerland) diluted in 2.5 ml of sterile saline solution; or 3 doses of Albuterol (US $ 6.73) every twenty minutes for one hour (Glaxo Smith Kline Albuterol ampoules, 2.5 mg/2.5 ml, at a dose of 0.15 mg/kg/dose, maximum dose 2.5 mg). Symptoms score, oxygen saturation and lung function testing were recorded before and one hour after commencing treatments.
ResultsBoth groups improved significantly on all parameters, except for FEV 1 in the Albuterol group.
ConclusionsSingle dose nebulised Formoterol Fumarate (dry powder) in sterile saline solution, as depicted in this trial, is equivalent to three doses of Albuterol every twenty minutes for one hour in acute asthma in children, simplifying acute care management and at one fifth of medication costs. A pursuit of simpler and more cost effective approaches is found wanting in developing nations with depressed economies and unique cultural and socio-medical contexts; also, in countries where pharmaco-economics orients quality of health policies, novel approaches like this are worth exploring.
Asthma faces tremendous challenges in developing nations and the Global Initiative for Asthma (GINA), well aware of these facts, encourages adaptations to particular needs; aspects of diagnosis, treatment and access to medication are but a few1. Acute asthma care has risen alarmingly for the last twenty years registering more than a million visits per year to the ambulatory network of the Ministry of Health of Venezuela, serving 80 % or more of a mostly urban and impoverished population of 26 million inhabitants2; this high morbidity falls second to entities like the “viral syndrome”, although ahead of diarrheal diseases and others. Similar situations are confronted by other developing nations and when compared, for example, to reports from the United States with roughly ten times the population and only twice the morbidity, the nature of the problem is underscored2.
Across the nation and for many years, delivery of this acute care has been in nebulised form, widely employing a fixed ipratoprium bromide/fenoterol combination (Berodual®, Boehringer-Ingelheim) in repeated dosing, thus allowing profitable use of our ample availability of nebulization devices2. Inhalation of Formoterol dry powder, a long acting beta-agonist with implicit immediate bronchodilator effect, has been found as effective as the short acting beta-agonists in the treatment of acute asthma as well as in rescue or needed use in asthma3–7; when used in a daily maintenance dose of 24μg for a year (Foradil®, Aerolizer®), no serious adverse effects have been detected in children8. However, very little information is available in relation to the use of nebulised Formoterol in the acute setting, a form of administration more suitably adapted to our medical and cultural context9–12. Recently (2007), the Federal Drug Administration (FDA), approved a Dey L.P. 20μg/vial of Formoterol Fumarate saline solution (Perforomist®) for maintenance use in COPD13,14; furthermore, over a period of approximately 10years we have developed an expertise in the use of nebulised Formoterol powder (Foradil®, Novartis Pharma AG, Basel Switzerland) in sterile saline solution9–12, a non-conventional form of administration found highly efficacious in relieving bronchospam when freshly prepared and immediately administered. This dilution of Foradil® has no influence on saline pH or osmolality and no differences are detected under the 40 × microscope (Tania Aguirre PhD, Investigaciones Especiales, Centro Medico Docente La Trinidad, Caracas, Venezuela); also microbiologic cultures, in standard media, have shown no growth of microorganisms (Departamento de Microbiologia, Laboratorio Autoanalitico del Instituto de Clinicas y Urología Tamanaco, Caracas, Venezuela) and its immediate administration after preparation obviates any practical considerations about stability. Our mentioned long expertise9–12 prompted us to carry out this study, blindly comparing a nebulised single dose of Formoterol Fumarate powder (two Foradil® 12μg capsules, 24μg in 2.5ml of sterile isotonic saline solution) vs. 0.1 % nebulised Albuterol ampoules (2.5mg/ 2ml solution, Glaxo Smith Kline) every twenty minutes, for one hour, in acute asthma in children.
METHODOLOGYThis is a prospective double-blind randomised controlled trial in children aged 5 to 12years with acute asthma exacerbations, between January 2006 and March 2007, admitted to the emergency room of the Hospital Pediátrico “Elias Toro” serving an impoverished area of Caracas. Eligibility criteria included a previous doctor's diagnosis of asthma in accordance with standard criteria, ability to perform a spirometric manoeuvre in the sitting position under enthusiastic coaching (Vitalograph® Alpha Spirometer) and absence of fever with no clinical evidence of superimposed bacterial infection (purulent bronchitis, otitis media, rales). The protocol was approved by the Institutional Review Board and Ethics Committee of the participating Institution and parent(s) or responsible party signed an informed consent before admission in study.
Children were excluded if presented with a first wheezing episode or one or more of coexisting cardiac, renal or other chronic pulmonary diseases.
After an initial medical evaluation by one of two research pediatricians (ER, VV), a trained nurse not related to the study, randomly assigned patients by a computer generated program, to receive either 3 doses of nebulised Albuterol every 20 minutes for one hour or a single dose of nebulised Formoterol Fumarate; final medical evaluations were performed by either research physician one hour after commencing treatments and neither pediatricians nor patients were aware of treatments received.
Treatments were prepared separately by the same nurse consisting of nebulised 0.1 % Albuterol ampoules (0.15mg/kg/dose, maximum 2.5mg/2.5ml) every 20 minutes for one hour or a single dose of nebulised Formoterol Fumarate powder (two Foradil® 12μg capsules, for a total of 24μg diluted in 2.5ml of sterile isotonic saline solution, freshly prepared and immediately administered), delivered via a PARI® PRONEB® turbo Nebulizer (Compressed Air without Oxygen) with face mask and allowing for a maximum nebulization time close to 10 minutes. Symptoms score (Modified Wood Downes's score, Table I), O2 saturation (Welch Allyn) and the best of 3 spirometric efforts (FEV1 and Peak Flow), at beginning and end of treatments (one hour), were considered for analysis; notes were taken of any medications administered in the previous 12 hours.
Wood-Downes score modified by authors*
| 1 | 2 | 3 | |
| Respiratory Rate | Normal or up to 30 % > than mean for age | 30-50 % > than mean respiratory rate for age | > 50 % than mean respiratory rate for age |
| O2 Saturation | > 95 % in room air | 92-95 % in room air | < 92 % in room air |
| Auscultation | Normal or moderate wheezing at the end of expiration | Unequal breath sounds and intense wheezing during expiration | Breath sounds reduced and intense wheezing during inspiration or silent chest |
| Use of accessory muscles | No | Intercostals, subcostals (mild to moderate) | Intercostals, subcostals, suprasternals (intense) or paradoxical breathing |
| Conscious | Normal | Agitated | Agitated/Somnolence |
| PEF (% of Predicted) | 70-100% | 50-70% | < 50 % |
The sample size needed to detect meaningful statistical differences between comparison groups, with alpha = 5 % and beta = 20 %, is equal to 25 evaluable subjects in each group.
Homogeneity of variance test (Levene's test) showed that values obtained were not normally distributed, so, non-parametric tests were employed.
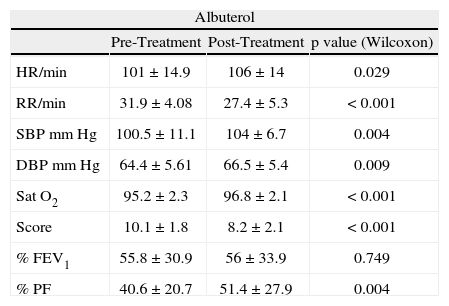
RESULTSDuring the study period, 50 children were randomised and enrolled in the trial. One child in the Albuterol group, due to worsening asthma, was unable to complete the pulmonary function test at the end of treatment. There were no significant differences between groups for age, gender, height, weight, previous relevant medication or tobacco exposure. Significant improvements were noted for all parameters (Table II), except for FEV1 in the Albuterol group.
Comparison between pre-treatment and post-treatment values
| Albuterol | |||
| Pre-Treatment | Post-Treatment | p value (Wilcoxon) | |
| HR/min | 101 ± 14.9 | 106 ± 14 | 0.029 |
| RR/min | 31.9 ± 4.08 | 27.4 ± 5.3 | < 0.001 |
| SBP mm Hg | 100.5 ± 11.1 | 104 ± 6.7 | 0.004 |
| DBP mm Hg | 64.4 ± 5.61 | 66.5 ± 5.4 | 0.009 |
| Sat O2 | 95.2 ± 2.3 | 96.8 ± 2.1 | < 0.001 |
| Score | 10.1 ± 1.8 | 8.2 ± 2.1 | < 0.001 |
| % FEV1 | 55.8 ± 30.9 | 56 ± 33.9 | 0.749 |
| % PF | 40.6 ± 20.7 | 51.4 ± 27.9 | 0.004 |
| Formoterol | |||
| Pre-Treatment | Post-Treatment | p value (Wilcoxon) | |
| HR/min | 98.2 ± 12.4 | 99.3 ± 13.5 | 0.338 |
| RR/min | 30.8 ± 6 | 24.88 ± 4.89 | < 0.001 |
| SBP mm Hg | 102.2 ± 8.93 | 104.8 ± 11.2 | 0.078 |
| DBP mm Hg | 66.3 ± 5.9 | 67.7 ± 5.3 | 0.134 |
| Sat O2 | 94.5 ± 1.7 | 96.9 ± 1.8 | < 0.001 |
| Score | 10.5 ± 1.2 | 7 ± 1.7 | < 0.001 |
| % FEV1 | 58 ± 31.7 | 67.8 ± 31.1 | < 0.001 |
| % PF | 47.2 ± 20.3 | 57.6 ± 24.5 | < 0.001 |
HR: heart rate; RR: respiratory rate; SBP: systolic blood pressure; DBP: diastolic blood pressure; Sat O2: oxygen saturation; Score: clinical symptom score (modified from Downes); FEV1: maximum expiratory flow in one second % of predicted value; PF: peak flow: maximum peak flow% of predicted value.
The present worldwide tendency at the acute setting is to replace nebulised medications with either Metered Dose Inhalers (MDIs, with or without the use of spacers) or with medication in powder form1,17. Venezuela has developed a pattern of nebulised acute care over many years and changing it will entail, among other endeavours, intense and correspondingly widespread education efforts; also, a most likely future underutilisation of already existing nebulisation devices. Such an approach is not foreseeable to us, as a public health policy, in the very near future.
Considering the ample use of these nebulisation devices at ambulatory facilities of the Ministry of Health of Venezuela and, since Berodual® is not a widely employed therapy for acute asthma in many countries, we compared nebulised Formoterol Fumarate powder with current Albuterol recommendations. No doubt nebulisations can be used by persons of any age or with lack of coordination, thus increasing the therapeutic range of a drug, something also to bear in mind. Formoterol Fumarate dry powder at variable and multiple dosing has been compared to frequent and different doses of Albuterol in powder and/or in MDI form, and found as effective for acute bronchospasm3–7; at the 24μg dry powder daily maintenance dose (Foradil®, Aerolizer®) it has shown no serious adverse effects in children8. However, very little information exists for nebulised Formoterol Fumarate powder in acute asthma9–12, though an inhalation saline solution of 20μg/vial of Formoterol Fumarate for BID nebulisation in COPD has been recently developed by Dey L.P. (Perforomist®) and approved (2007) by the FDA13,14. Our high burden of acute asthma care2, the known fact that the greater expenditures for asthma come from hospitalisations and emergency visits1, our long and previous experience in the use of this non-conventional form of administration9–12, the entailed management simplification and significant cost savings (LOCATEL® chain drugstore, October 2007: Foradil® 2 capsules $ 1.35 vs. 3 ampoules of Albuterol GSK $ 6.73), and the fact that, to our knowledge, no available study has compared nebulised Formoterol Fumarate powder with nebulised Albuterol, all prompted us to carry out this study. Children with acute asthma experience difficulties with pulmonary function tests15; however, these functional measurements of airway obstruction do not necessarily correlate with other parameters, since different aspects of the disease are under scrutiny16. The lack of significant FEV1 improvement found after three doses of Albuterol in this study might very well fall under these premises and if higher doses were to be employed on such a heterogenous population, perhaps a better response would be found. As depicted in this trial, nebulised Formoterol Fumarate powder seems to be equivalent to 3 doses of nebulised Albuterol in children's acute asthma. A pursuit of simpler but also more cost-effective approaches is found wanting in developing nations with depressed economies and unique cultural and social contexts, but it is also worth considering for more affluent societies where pharmaco-economics approaches guide health budgets17. To orient public health policies and efficiently impact management of acute asthma, findings from this study need to be replicated in larger number of patients. In view of the reported controversial subsensitivity to Albuterol after employing long-acting bronchodilators, concomitant use of steroids should always be considered18.