To the Editor:
Pseudoephedrine is a sympathomimetic drug widely used in over-the-counter anti-catarrhal preparations. Despite its widespread use, cutaneous adverse effects are rare, and generally not life-threatening.
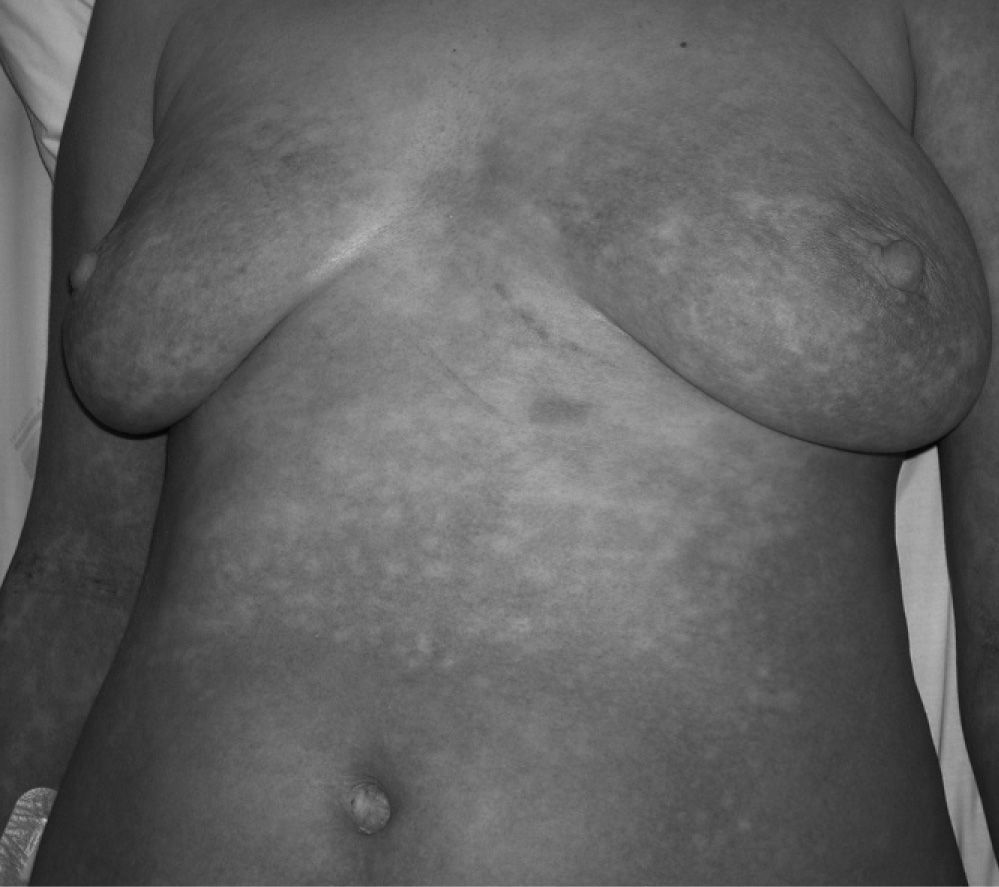
A 30-year-old Caucasian woman presented with a generalised, maculopapular, pruriginous dermatitis with facial oedema, malaise and fever (Fig. 1). During the five days preceding the emergence of the cutaneous lesions, the patient had been taking Actifed® (pseudoephedrine and triprolidine) for rhinorrhea and nasal congestion with no other accompanying symptoms. She denied previous intake of this drug.
Physical examination revealed a generalised, maculopapular dermatitis, sparing the distal extremities and mucous membranes, with facial oedema. Tympanic temperature was 38°C. No peripheral lymph nodes were palpable and hepatosplenomegaly was absent.
Laboratory tests revealed slight neutrophilia (7.7 × 109 cells/L) without leucocytosis and elevated C-reactive protein levels in blood (21.2mg/dL). Mycoplasma pneumonia, Epstein Barr, Cytomegalovirus, B and C hepatitis, HIV-1 and HIV-2 serologies were negative. Antistreptolysin titter was negative. Chest radiography showed clear lung fields.
Histopathological examination revealed vacuolar degeneration of the basal layer, oedema and haemorrhage of the papillary dermis and mixed perivascular inflammatory infiltrate.
The patient's condition was successfully managed with oral prednisolone.
Patch tests performed with Actifed® as it is and with pseudoephedrine sulphate (1 % pet) were both positive (++). Portuguese standard series, ephedrine (1 % pet) and phenylephrine (1 % aq) were negative. We were unable to test for triprolidine. Testing pseudoephedrine sulphate (1 % pet) elicited no reactions in five healthy controls.
Pseudoephedrine-induced cutaneous adverse effects are uncommon. Fixed drug eruption accounts for the majority of the cases. However generalised papular and papulovesicular eruption, erythroderma, systemic contact dermatitis, acute generalised exanthematous pustulosis, pseudo-scarlatina and recurrent toxic shock syndrome have also been described1–6.
Pseudoephedrine belongs to the phenylamine family of compounds and shares with ephedrine a common phenylpropanolamine skeleton. Phenylephrine is also a phenylamine but has a phenylethanolamine skeleton. Allergic contact dermatitis has been reported to phenylephrine eye-drops 7 and ephedrine containing anti-catarrhal preparations8. Given that cross-reaction between these two drugs and pseudoephedrine has been described previously 9 it would be prudent to patch test patients whenever pseudoephedrine allergy is suspected. No restrictions were made concerning other sympathomimetic drugs in our patient as no cross-sensitivities were found.