To the Editor:
Snail consumption is frequent in the Mediterranean area as a delicacy, mostly in Portugal, Spain, Italy and France1. It has been recognized as a food allergy with a significant relation with house dust mite (HDM) sensitisation (associated or not with respiratory symptoms). This fact is usually due to cross-sensitisation to the major allergen, tropomyosin, present in HDM, seafood, snail and cockroach2,3. Snail allergy can induce several clinical presentations namely asthma, urticaria, angio-oedema, anaphylaxis or even death1. There have been very few case-reports of isolated snail allergy, in the absence of HDM sensitisation.
The authors report a 49year old man, who was admitted to the Immunoallergology Department in 2006 with a first episode of bilateral eyelid oedema, itchy lip and throat, and dysphonia thirty minutes after snail ingestion, although denying either urticaria or dyspnoea. He had no history of respiratory symptoms (namely rhinitis or asthma) suggestive of HDM or any other aeroallergens allergy; he also denies prior food allergy.
The patient was submitted to skin prick tests (SPT) with HDM and other aeroallergens namely cockroach, which were all negative. SPT with snail extract (Helix aspersa- Bial Aristegui®) and prick-to-prick with cooked and raw snail were positive and negative to all other food allergens, namely shrimp, crab, lobster, clams, cuttlefish, and squid. Specific IgE (Unicap-Phadia®) to HDM and tropomyosin were negative (< 0.35 kUA/L) and positive to snail-Helix aspersa (0.89 kUA/L – class II). Total IgE was 66.0 kU/L.
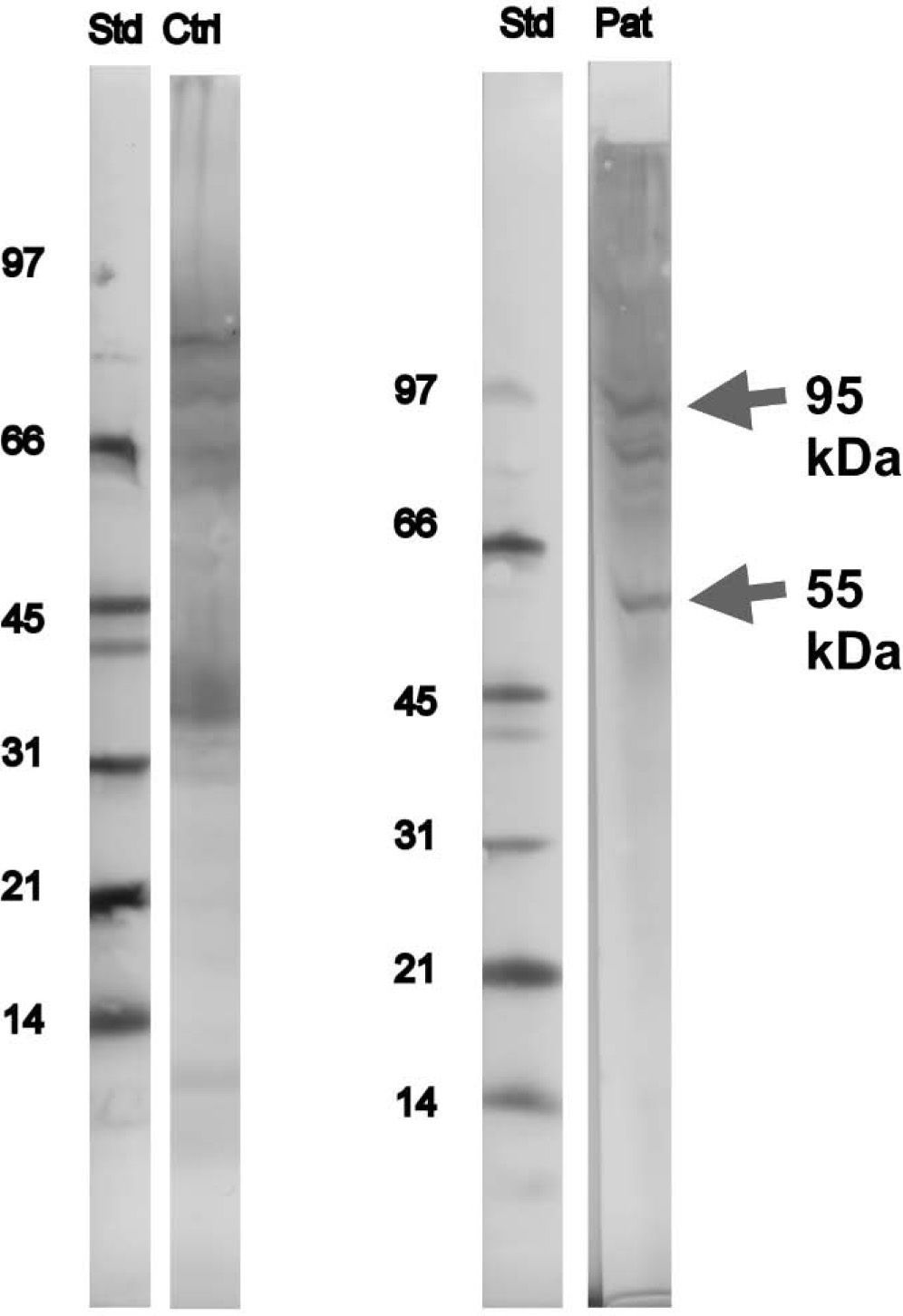
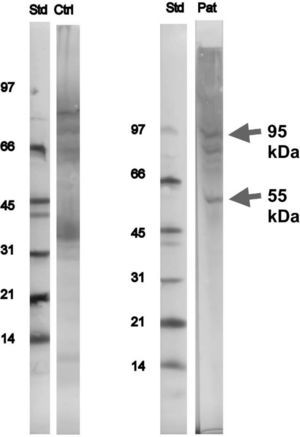
Immunoblotting and inhibition tests of serum sample from the patient were performed in Sweden (MIAB-Phadia®), using Novex® (USA) XCell miniCell, Novex® nitrocellulose blotting membranes and NuPAGE 4–12 % running buffer and rabbit anti-IgE, biotin labelled, in-house, MIAB®. Other equipment used was biotin labelled protein standard, low molecular weight range (phosphorylase b 97 kD, bovine serum albumin 66KD, ovalbumin 45 kD, carbonic anhydrase 31 kD, soybean trypsin Inhibitor 21 kD, Lysozym 14 kD), colorimetric development (BCIP/NBT), BioRad and streptavidin-alkaline phosphates, Zymed®, USA. Two bands, a distinct one at 55 and a faint one at 95kDa were identified, which were not paralleled by similar bands on the in-house control sample (in-house human pool of sera from snail sensitised patients and our patient samples were immunoblotted against SDS-PAGE separated serial dilutions of extracts from f314 snail, MIAB), i.e. they were specific for the patient (Fig. 1).
The absence of HDM sensitisation was confirmed with the determination of native protein of Dermatophagoides pteronyssinus (n Der p1) and tropomyosin which was negative. Lack of HDM sensitisation was also supported by inhibition tests, where the IgE binding to snail was inhibited by snail extract but not by the HDM extract.
The peculiarity of this case-report is based on the fact that snail allergy without cross-sensitisation with HDM is very uncommon. Some authors have described some bands of this species of snail (Helix aspersa), but in this patient one (55kDa) and probably two (95 KDa) new bands were identified, not referred in the literature until now. According to their molecular weight we can say that they are different from the already described Helix aspersa major allergen (Hel a), which is a myosin protein, and weights over 208kDa, but we cannot tell what kind of proteins these are. They are, probably, minor allergens possibly responsible for snail allergy in this patient. In order to elucídate the importance of this protein, it will be useful to perform immunoblotting in further patients with isolated snail allergy.