Exercise-induced bronchoconstriction (EIB) has a high prevalence in children with asthma, and this is a common problem, even in case of controlled asthma, because of the high levels of physical activity in the childhood.
ObjectivesThe aim of our study was to identify factors associated with the development of EIB in children with controlled asthma.
MethodsWe studied children evaluated for asthma. A personal and familiar history was collected from each patient to estimate asthma severity, precipitating factors, exercise ability, immunotherapy treatment and atopic familiar disorders. Skin prick tests for inhalant allergens, pulmonary function tests (PFTs) and exercise challenge test (ECT) measurements were realized in every patient. We used the Chi Squared test to compare qualitative variables, the Student's-t test for quantitative variables and a logistic regression analysis to estimate the independent effect of the variables.
ResultsWe evaluated 132 asthmatic patients. Eighty-two, 6 to 14 years old (average 110±36.9 months), were included in the study. Forty one have coughing or wheezing with exercise at least three months ago, in addition to a positive ECT; 9 of these children had solitary EIB (group A), and 32 (group B) had controlled chronic asthma, 27 intermittent and 5 moderately persistent. Forty one controlled asthmatic children, 39 intermittent, 1 mildly persistent and 1 moderately persistent (group C) had a good tolerance for exercise with a negative ECT. No differences were found in familiar history, asthma severity or evolution time in B vs C group.
We found that 35 patients (42,68 %) patients were sensitized to indoor allergens: 24 (58,53 %) were patients suffering EIB and 11 (26,8 %) allowed to group C. Precipitating factors of asthma were in group B: respiratory infections in 19 cases, pollen in 20 and in 10 indoor allergens exposure. In group C: 14 patients had asthmatic symptoms with viral respiratory infections, 32 with pollen and 2 with indoor allergens exposure. A patient from group A had allergy rhinitis after exposure to cats. Allergy to indoor allergens demonstrated an direct association to EIB suffering (p=0,026).
Twenty six patients with allergic asthma followed pollen immunotherapy treatment, 7 of group B (33,3 %) and 19 (59,3 %) of group C. This treatment was inversely associated with EIB suffering (p=0,048). A logistic regression analysis confirmed the independence of both variables as predisposing and protecting factors in EIB suffering.
ConclusionsAllergy to indoor allergens might be considered a risk factor for EIB. Immunotherapy treatment could be a protective factor against the development of EIB in children with allergic asthma.
Exercise-induced asthma or exercise-induced bronchoconstriction (EIB) is typically characterised by a history of coughing or wheezing, or a history of shortness of breath with exercise. EIB is the result of a post-exercise airway obstruction with a reduction in forced expiratory volume in 1 second (FEV1) of greater than 10 % compared with pre-exercise values -with 5 minutes of exercise at 85–90 % of maximum- and relief of post-exercise airway obstruction with inhaled-agonist.
The mechanisms of EIB have not fully been elucidated. While exercise may provide multiple stimuli for induce bronchoconstriction, hyperpnoea is the dominant stimulus for inducing EIB. Cooling and drying, and possibly re-warming affect airways resulting in the release of multiple local inflammatory mediators of which histamine, prostaglandins and, especially leukotrienes are important. Airway narrowing occurs post-exercise as a result of the mediator release, which is possibly produced along with warming of the airway, resulting bronchoconstriction, vascular engorgement and leakage, and increased mucus production.
The prevalence of EIB in asthmatics has been reported to range from 40 % to 90%1,2. Even in asymptomatic asthmatic children, exercise is the most common stimulus for inducing airway obstruction and then EIB is an important problem because of the high levels of physical activity in childhood.
The purpose of this study was to analyze personal characteristics, precipitating factors of asthma, asthma severity and immunotherapy treatment in our asthmatic children population and its relation with EIB. Our aim is to identify factors associated with EIB development in children with controlled asthma.
METHODSPopulation studiedWe studied all children evaluated in our office for asthma from June to October. Children from 6 to 14years old, with a diagnosis of asthma and demonstrated ability to perform lung function and the challenge exercise test, were included in this study. Patients with cardiac disease were excluded from the study.
The presence of asthma was based on criteria such as those presented by the American Thoracic Society (ATS) or the European Respiratory Society (ERS). Precipitating factors (exercise, allergens, and immunotherapy treatment) were evaluated. A clinical history in order to estimate the symptoms, the severity and the precipitating factors of asthma (viral infections, allergens exposure, exercise), eczema or food allergy and familiar atopy were all recorded for all children and their parents. In addition, a physical examination, skin tests with aeroallergens and pulmonary function tests (PFTs) measurements with an exercise challenge test, were performed on all patients to confirm the diagnosis. The control of asthma was assured if the patient did not have any different asthmatic symptoms other than EIB for at least the three previous months, didn't experience any daytime symptoms more than twice a week, didn't demonstrate any nocturnal symptoms and didn't need any rescue treatment more than twice a week. Moreover, the lung function basal value FEV1 of each child was equal or superior to the predicted 80%.
The procedures used on patients have been done after obtaining informed consent.
Diagnostic testingSkin prick tests (SPTs) were performed on all patients with glycerinated allergens extract (ALK-Abelló, Madrid, Spain) of important local pollens (lolium perenne, cynodom dactylon, olea europaea, platanus acerifolia, plantago lanceolata, artemisia vulgaris, cupressus arizonica), house dust mites (dermatophagoides pteronyssimus, dermatophagoides farinae), common fungi (Aspergillus, Alternaría, Cladosporium) and animal dander (dog, cat, rabbit, horse and hamster). Glycerosaline solution and histamine dihydrochloride at 1 % were applied as negative and positive controls. A net weal diameter 3mm larger than the one produced by the negative control was considered positive.
Pulmonary function tests (PFTs) and exercise challenge test (ECT)PFTs measurements were taken with a Master Screen spirometer (Erich Jaeger, Würtzburg, Germany). Once resting PFTs had been taken and the results were determined to be normal, an exercise test was conducted on a motor-driven treadmill, following guidelines for the exercise challenge test ATS3. A diagnosis of EIB was established when a child had a positive exercise challenge test with a reduction in FEV1 of greater than 15 % compared with pre-exercise values, and relief of post-exercise airway obstruction with inhaled-agonist. A diagnosis of asthma without EIB included a negative history with exercise and a negative exercise challenge test.
Data analysisWe used Chi Squared test to compare qualitative variables, the Student's-t test for quantitative variables and a logistic regression analysis to estimate the independent effect of the variants.
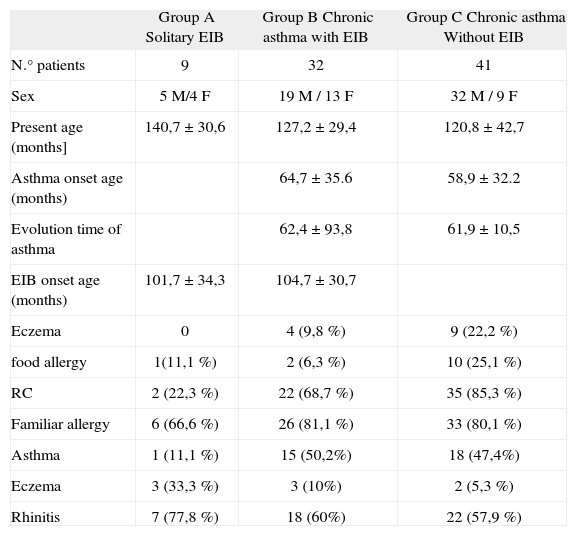
RESULTSWe evaluated 132 patients for asthma, from 6 to 14years old. Fifty children were excluded from the study (31 had uncontrolled asthma and 19 did not demonstrate ability to perform spirometry or an exercise challenge test). Eighty-two children, 56 males (68.3 %) and 26 females (31.7 %), with a diagnosis of controlled asthma were included in this study. They were mean aged to 125.49 ± 36.9months. Forty-one patients referred coughing, wheezing or dyspnea with vigorous physical activity. Nine patients had solitary EIB with a positive ECT (group A). Seventy-three children had chronic asthma (66 intermittent, 1 mildly persistent and six moderately persistent). Their asthma started between 8 and 82months of age with a mean of 61.42 ± 33.64months. Only thirty-two of these patients referred coughing, wheezing or dyspnea with vigorous physical activity, and all of them had a positive ECT, they were called group B. The other 41 children had chronic asthma without coughing or wheezing with exercise and everyone in this group had a negative ECT (group C). Fifty-nine children had eczema or food allergy and 65 had a familiar history of allergy. There were no differences between groups in asthma severity, sex, present age, asthma onset age, EIB onset age, evolution time of asthma, eczema or food allergy. Atopic familiar disorders did not demonstrate differences except to familiar eczema group A vs group C (P = 0.046) (Table I).
Characteristics of the asthmatic groups. We found no differences between groups in these variables except to familiar eczema group A vs group C (P = 0.046)
| Group A Solitary EIB | Group B Chronic asthma with EIB | Group C Chronic asthma Without EIB | |
| N.° patients | 9 | 32 | 41 |
| Sex | 5M/4F | 19M / 13F | 32M / 9F |
| Present age (months] | 140,7 ± 30,6 | 127,2 ± 29,4 | 120,8 ± 42,7 |
| Asthma onset age (months) | 64,7 ± 35.6 | 58,9 ± 32.2 | |
| Evolution time of asthma | 62,4 ± 93,8 | 61,9 ± 10,5 | |
| EIB onset age (months) | 101,7 ± 34,3 | 104,7 ± 30,7 | |
| Eczema | 0 | 4 (9,8 %) | 9 (22,2 %) |
| food allergy | 1(11,1 %) | 2 (6,3 %) | 10 (25,1 %) |
| RC | 2 (22,3 %) | 22 (68,7 %) | 35 (85,3 %) |
| Familiar allergy | 6 (66,6 %) | 26 (81,1 %) | 33 (80,1 %) |
| Asthma | 1 (11,1 %) | 15 (50,2%) | 18 (47,4%) |
| Eczema | 3 (33,3 %) | 3 (10%) | 2 (5,3 %) |
| Rhinitis | 7 (77,8 %) | 18 (60%) | 22 (57,9 %) |
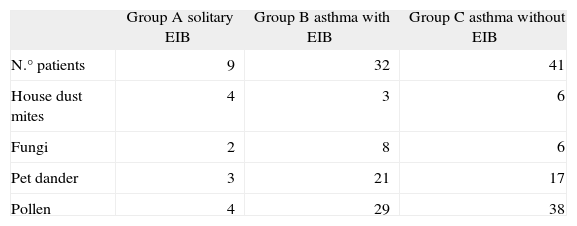
Most patients had multiple specific aeroallergen sensitivities: 13 children were sensitized with house dust mites, 16 with fungi, 41 with pet dander, and 71 with pollens (Table II).
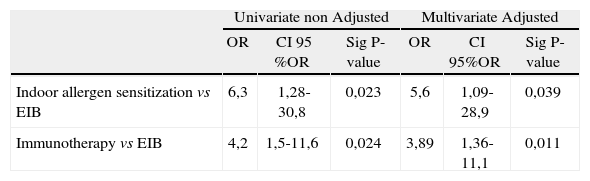
The analysis of the EIB patients (groups A,B) vs group C demonstrated a clear association between EIB suffering and the sensitization to indoor allergens (p = 0,023); OR 6,3 (1,28-30,8).
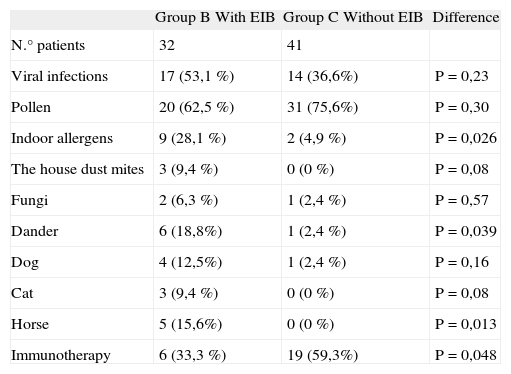
The factors underlying asthma exacerbation were viral respiratory infections in 31 children; pollen exposure in 52; indoor allergen exposure in 11 patients (dust mites in 3 cases, moulds in 2 cases and dander in 7 children). Indoor allergen allergy was associated with EIB (p < 0,026).
Twenty-five patients had been treated previously with immunotherapy, 6/20 (33,3 %) children with pollen allegy in B group and 19/32 (59,3 %) in C group. The immunotherapy treatment showed a negative association with EIB (p = 0,024); OR 4,2 (1,5-11,6) (Table III).
Precipitating factors of asthma and immunotherapy treatment in B and C groups of chronic asthmatic children
| Group B With EIB | Group C Without EIB | Difference | |
| N.° patients | 32 | 41 | |
| Viral infections | 17 (53,1 %) | 14 (36,6%) | P = 0,23 |
| Pollen | 20 (62,5 %) | 31 (75,6%) | P = 0,30 |
| Indoor allergens | 9 (28,1 %) | 2 (4,9 %) | P = 0,026 |
| The house dust mites | 3 (9,4 %) | 0 (0 %) | P = 0,08 |
| Fungi | 2 (6,3 %) | 1 (2,4 %) | P = 0,57 |
| Dander | 6 (18,8%) | 1 (2,4 %) | P = 0,039 |
| Dog | 4 (12,5%) | 1 (2,4 %) | P = 0,16 |
| Cat | 3 (9,4 %) | 0 (0 %) | P = 0,08 |
| Horse | 5 (15,6%) | 0 (0 %) | P = 0,013 |
| Immunotherapy | 6 (33,3 %) | 19 (59,3%) | P = 0,048 |
There were statistical differences about indoor allergens as precipitating factors and immunotherapy treatment as a protector factor for EIB between both asthmatic children groups
The multivariable logistic regression analysis confirmed that the indoor allergen sensitization and the immunotherapy treatment were independent factors (Table IV).
Univariable and multivariable statistical regression analysis of sensitization indoor allergens and immunotherapy treatment in asthmatic children and association with EIB
| Univariate non Adjusted | Multivariate Adjusted | |||||
| OR | CI 95 %OR | Sig P-value | OR | CI 95%OR | Sig P-value | |
| Indoor allergen sensitization vs EIB | 6,3 | 1,28-30,8 | 0,023 | 5,6 | 1,09-28,9 | 0,039 |
| Immunotherapy vs EIB | 4,2 | 1,5-11,6 | 0,024 | 3,89 | 1,36-11,1 | 0,011 |
The results of the multivariable logistic regression analysis confirmed indoor allergen sensitization as a risk factor to EIB and the immunotherapy treatment as a protector factor in the development of EIB.
Post exercise bronchospasm is a well-known phenomenon, particularly affecting children with asthma. It is an important problem due to the high levels of physical activity in childhood. Participation in sporting activities is an aim in the management of asthma in children and moreover, exercise tolerance is an important measure of asthma control and should be monitored by exercise testing. Then, recognition of predisposing factors in EIB is the first step in treatment of this condition.
We found that children with allergy or even only sensitization without a clear bronchial response after exposition to indoor allergens had a greater probability of developing EIB. This result could ensure the participation of exposure to allergens in EIB, even when the level of exposure to allergens alone is not enough to develop bronchoconstriction. It is recognized that airway inflammation plays a central role in the pathogenesis of asthma, but how it relates to exercise-induced bronchoconstriction (EIB) is not completely understood. Various studies have investigated the relationship between EIB and baseline concentrations of inflammatory markers in exhaled condensated breath and induced sputum. The baseline concentrations of inflammatory markers are higher in asthmatic children with EIB4,5. The exposure to indoor allergens may favour the development of EIB. Exercise-induced bronchoconstriction has been associated with eosinophilic airway inflammation, bronchial hyper responsiveness (BHR), atopy and airway obstruction. There is overwhelming evidence that respiratory allergies are a major factor in the pathogenesis of asthma in children. Removing offending pets and limiting exposure to relevant indoor allergens such as dust mites or mould proteins may lead to a reduction in asthma symptoms and bronchial hyper responsiveness with improvement of EIB and a reduction of asthma medications6–8. However, a minimum exposure might maintain the bronchial inflammation and could be a predisposing factor in developing EIB.
Some studies show that EIB is common in children with eczema9. We found no differences related to eczema between asthmatic children with or without EIB, but the patients with solitary EIB had a greater proportion of eczema in their family. At the moment we do not have an explication for this finding, and other studies are necessary to confirm this result.
On the other hand, in our study we only had a small proportion of asthmatic children with EIB who had immunotherapy treatment in contrast to group C without EIB. Immunotherapy treatment appears as a protector factor for EIB in our asthmatic children. Numerous studies have shown an improvement in asthmatic allergic children with immunotherapy and our results support those conclusions10–13). Two meta-analysis conducted by Abramson et al concluded that subjects randomized to immunotherapy for asthma treatment showed improvement in asthma symptoms and required less asthma medication. In addition, a reduction in both non-specific and allergen-specific bronchial reactivity was demonstrated, compared to those randomized to placebo14,15. These results confirm the decrease of bronchial inflammation in allergic asthma after immunotherapy, and in the end a minor response to exercise.
Following an analysis logistic regression we were able to confirm the independence of both variables, allergy to indoor allergens and immunotherapy treatment as predisposing and protector factors in the development of EIB in children. Children with sensitization to indoor allergens had developed EIB in a proportion at least 5 times mayor than children without that sensitization and children with immunotherapy treatment demonstrated EIB in a proportion 3,8 times minor than those without this treatment.
Therefore, the asthma control plan should always include detailed instructions for the child and family to help minimize exposure to relevant allergens16,17 and immunotherapy treatment should be taken into account in the treatment of allergic asthmatic children.