T helper 9 (TH9) cells are considered as newly classified helper T cells that have an important role in the regulation of immune responses. Since these cells preferentially produce IL-9, these cells are termed TH9 cells. Recently, the role of TH9 and its signature cytokine (IL-9) has been investigated in a wide range of diseases, including autoimmunity, allergy, infections, cancer and immunodeficiency. Herein, we review the most recent data concerning TH9 cells and IL-9 as well as their roles in disease. These insights suggest that TH9 cells are a future target for therapeutic intervention.
CD4+ T cells have critical roles in controlling the immune responses and regulating the immune system. While recognizing a foreign antigen-derived peptide presented by major histocompatibility complex (MHC) class II, T cells are activated, leading to proliferation and differentiation into one of the several subsets of T-helper (TH) cells, including TH1, TH2, TH17, and inducible Tregs (iTregs). Each T cell subset exhibits a particular set of transcription factors and a unique pattern of cytokine production.1,2 Recently, another TH cell subset has been discovered that predominantly express interleukin-9 (IL-9) in the absence of other classical TH subset specific cytokines or transcription factors. This TH subset has been termed “TH9”.3,4 The cytokine IL-9 was first identified in the late 1980s by Schmitt et al. and was reported to be produced by activated T cells from mice.5 Subsequently, they described cytokines that were associated with the in vitro development of IL-9 producing TH cells.6 However, in addition to TH9 cells, several other cell types are also able to produce IL-9 namely TH2, TH17, regulatory T cells (Tregs), natural killer T cells (NKT cells), eosinophils, mast cells and innate lymphoid cell type 2 (ILC2s) demonstrating that IL-9 is not restricted to adaptive TH cells.7,8
IL-9 has been shown to be both harmful and beneficial for the immune system as recent studies have uncovered the role of TH9 cells and IL-9 in different diseases. In this review, we described the characteristics of TH9 cells and IL-9 and their differing roles in diseases including allergy, autoimmunity, infections, cancer and immunodeficiency.
Characteristics of TH9 and IL-9IL-9 and its receptorIL-9 has a role in the growth and promotion of T cells and mast cells, so it was initially named as T cell growth factor III (TCGFIII) and/or mast cell growth-enhancing activity (MEA).5,9 The IL9 gene is located on chromosome 13 in mouse and on chromosome 5 in human,10 with both the human and murine IL9 gene including five exons with 69% homology at the nucleotide level and 55% amino acid homology at the protein level.11
The IL-9 receptor is a heterodimer complex composed of IL-9Rα and common γ chain (γC). IL-9Rα is a specific receptor chain for IL-9, whereas the γC chain is also observed in other cytokine receptors including IL-2, IL-4, IL-7, IL-15, IL-21 and thymic stromal lymphopoietin receptors.12,13 In both human and mice IL-9R is expressed by on T cell lineages and effector T cells, with the exception of naive T cells.14,15 Among TH cell subsets, IL-9R is predominantly expressed on TH2 and TH17 cells.16 Several studies have indicated that multiple cell types can also express IL-9R including; macrophages, mast cells, dendritic cells, microglia, immature neurons, NK cells, NKT cells, Tregs, B cells (specially on germinal center B cells) and TH9 cells.17 Although the requirement of IL-9 function in non-hematopoietic cells is not yet known, IL-9Rα expression on cells including airway and intestinal epithelial cells, smooth muscle cells, and keratinocytes has been identified.18,8
IL-9 receptor signalingIL-9 exerts its functions through the interaction with the cell surface receptor IL-9R. Engagement of IL-9 with IL-9R causes the cross-phosphorylation of janus kinase (JAK) 1 and JAK3. This cross-phosphorylation activates signal transducer and activator of transcription (STAT) complexes, specifically STAT1 and STAT5 homodimers and STAT1–STAT3 heterodimers, leading to the upregulation of IL-9-induced gene transcription.19,20 It has also been reported that MAPK and insulin receptor substrate – PI3K pathways are activated through IL-9R signaling and play key roles in cell growth, survival, and differentiation.21,22
Cellular resources of IL-9IL-9 was first thought to be a TH2-specific cytokine,23,24 but was later shown to be predominantly produced by a distinct subset of T helper cells, termed “TH9”.3 TH2, TH17, Tregs, and TH9 are now considered as some of the main IL-9-producing cells within the immune system.18,23,25,26 TH17 cells secrete IL-17A and IL-17F, but can also express IL-9.16,27 Mouse models have indicated that both natural Tregs and inducible Tregs can also produce IL-9 under certain conditions,25 yet controversy remains regarding the production of IL-9 by human Tregs.28–30 In addition, NKT cells and eosinophils have also been reported to produce IL-9,31–33 and mast cells secrete IL-9 in the presence of IL-1 and LPS via activation of three discrete NF-kB binding sites within the IL9 promoter and subsequent gene transcription.34–37 Recently, in the lungs of allergen-sensitized mice, a papain-provoked model of airway inflammation demonstrated that ILC2s are the main source of IL-9 within the airway.38 In mice IL-18 can also stimulate IFNγ+ TH1 cells to produce IL-9.39,40
Cluster differential (CD) markers of TH9CD183 (CXCR3), CD193 (CCR3) and CD196 (CCR6) are chemokine receptors that are expressed on the surface of TH9 cells in humans. In contrast to other TH cell subsets (TH2, TH17 and TH22) TH9 cells do not express CD194+ (CCR4+) and CD294 (CRTH2).41–43 In a study of the collaborative immune regulation of TH9 cells and pleural mesothelial cells (PMCs) in human M. tuberculosis infection, Ye et al. showed that most TH9 cells express high levels of CD45RO in both tuberculous pleural effusion (TPE) and blood with low expression of CD45RA and CD62L indicating a memory phenotype. Pleural and blood TH9 cells express CCR7 and low levels of CCR2, CCR3, CCR4 and CCR5.44 In another study, Ye et al. demonstrated that IL-9 could strongly promote intercellular adhesion of human lung cancer cells to PMCs by upregulating expression of cellular adhesion molecules like ICAM-1, LFA-1, and VCAM-1 on both cancer cells and PMCs in vitro.45 It should be mentioned that the in vivo differentiation of TH9 cells is affected by multiple factors, therefore the effects of one or more cytokines on TH9 cell differentiation should be evaluated by in vitro experiments.
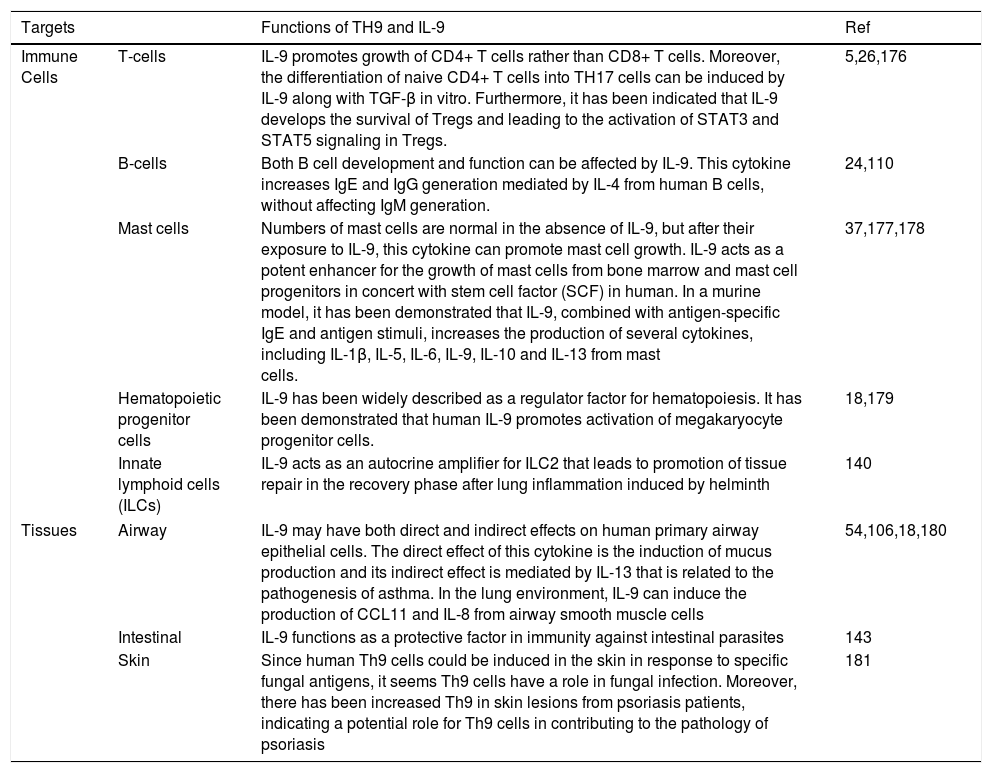
Functions of TH9 and IL-9IL-9 performs various positive and negative regulatory roles in the immune system. Functions of TH9 and IL-9 on different immune cells and tissues are summarized in Table 1.
Functions of TH9 and IL-9 on different immune cells and tissues.
| Targets | Functions of TH9 and IL-9 | Ref | |
|---|---|---|---|
| Immune Cells | T-cells | IL-9 promotes growth of CD4+ T cells rather than CD8+ T cells. Moreover, the differentiation of naive CD4+ T cells into TH17 cells can be induced by IL-9 along with TGF-β in vitro. Furthermore, it has been indicated that IL-9 develops the survival of Tregs and leading to the activation of STAT3 and STAT5 signaling in Tregs. | 5,26,176 |
| B-cells | Both B cell development and function can be affected by IL-9. This cytokine increases IgE and IgG generation mediated by IL-4 from human B cells, without affecting IgM generation. | 24,110 | |
| Mast cells | Numbers of mast cells are normal in the absence of IL-9, but after their exposure to IL-9, this cytokine can promote mast cell growth. IL-9 acts as a potent enhancer for the growth of mast cells from bone marrow and mast cell progenitors in concert with stem cell factor (SCF) in human. In a murine model, it has been demonstrated that IL-9, combined with antigen-specific IgE and antigen stimuli, increases the production of several cytokines, including IL-1β, IL-5, IL-6, IL-9, IL-10 and IL-13 from mast cells. | 37,177,178 | |
| Hematopoietic progenitor cells | IL-9 has been widely described as a regulator factor for hematopoiesis. It has been demonstrated that human IL-9 promotes activation of megakaryocyte progenitor cells. | 18,179 | |
| Innate lymphoid cells (ILCs) | IL-9 acts as an autocrine amplifier for ILC2 that leads to promotion of tissue repair in the recovery phase after lung inflammation induced by helminth | 140 | |
| Tissues | Airway | IL-9 may have both direct and indirect effects on human primary airway epithelial cells. The direct effect of this cytokine is the induction of mucus production and its indirect effect is mediated by IL-13 that is related to the pathogenesis of asthma. In the lung environment, IL-9 can induce the production of CCL11 and IL-8 from airway smooth muscle cells | 54,106,18,180 |
| Intestinal | IL-9 functions as a protective factor in immunity against intestinal parasites | 143 | |
| Skin | Since human Th9 cells could be induced in the skin in response to specific fungal antigens, it seems Th9 cells have a role in fungal infection. Moreover, there has been increased Th9 in skin lesions from psoriasis patients, indicating a potential role for Th9 cells in contributing to the pathology of psoriasis | 181 | |
Antigen-activated TH9 cells produce various cytokines in addition to IL-9. In mouse models, activated TH9 cells express high amounts of anti-inflammatory IL-10, and significantly lower levels of other cytokines including IL-17, IL-21, IL-22, and IFN-γ. However, TH9 cells do not express all of these cytokines simultaneously and require cytokine response which appears to vary depending on the cell microenvironment.46–49
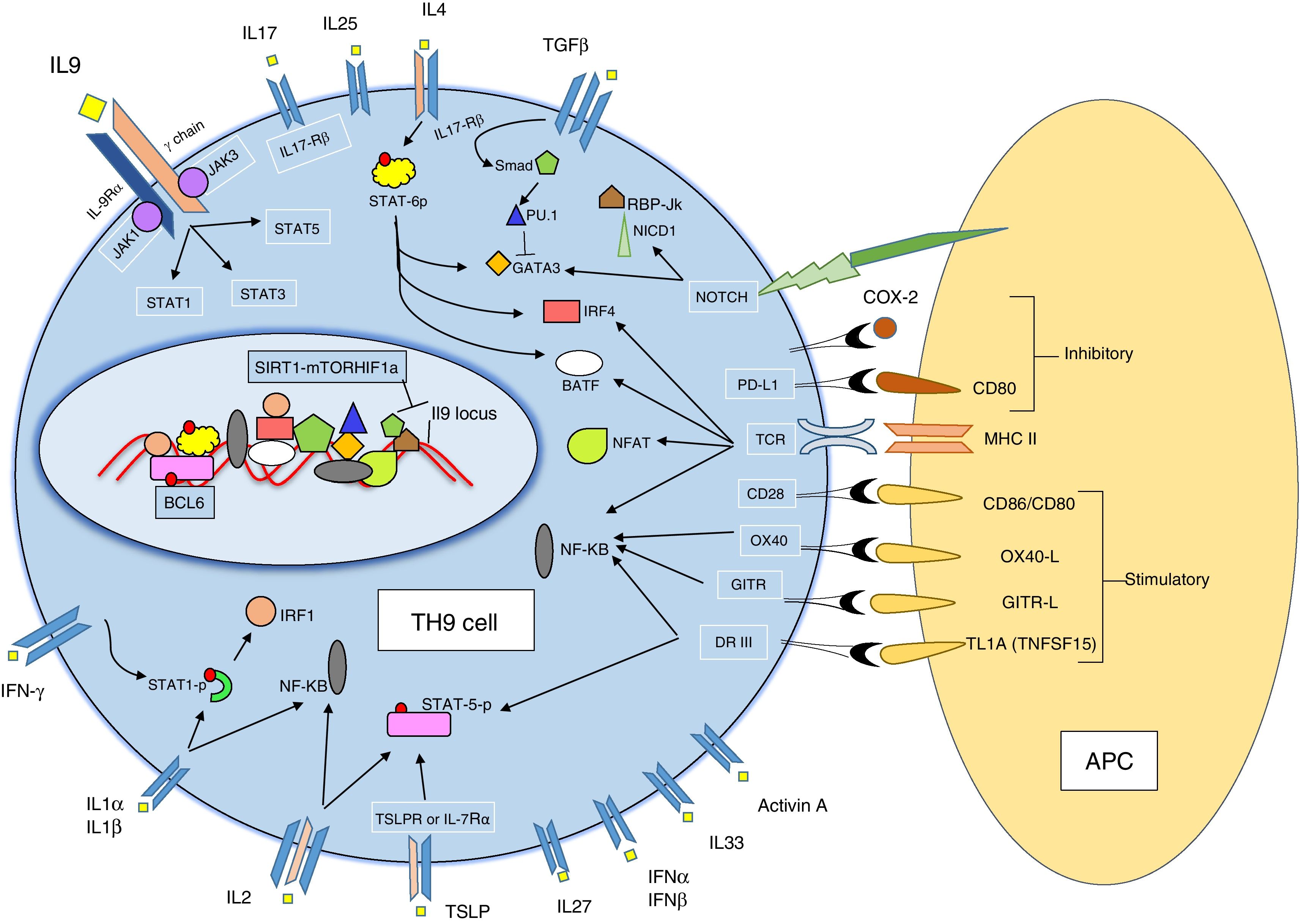
TH9 development and differentiationNaïve CD4+ T helper cells differentiate into effector T helper cell subsets in the presence of environmental elements such as cytokines and co-stimulatory factors. Each subset is described by a distinct pattern of cytokine secretion and function. In comparison to other characterized TH cell subsets (TH1, TH2, TH17 and iTreg), TH9 cells have been poorly described to date. Antigen-specific activation of naïve CD4+ T helper cells in the presence of transforming growth factor β (TGFβ) and interleukin-4 (IL-4) promotes the differentiation into effector TH9 cells3,6 suggesting that both of these cytokines are required for TH9 cell subset generation. Yet IL-4 and TGFβ cytokines can induce TH2 differentiation and Treg cell development, so additional co-stimulatory factors for TH9 generation (Fig. 1).18,50
Regulation of TH9 differentiation and IL-9 productionIL-9 is a signature cytokine for TH9 cell subset and secretion of this cytokine is regulated by the presence of some cytokines, co-stimulatory molecules and transcription factors (Fig. 1).
CytokinesIFN-γ. Schmitt et al. firstly demonstrated that IFN-γ inhibits the production of IL-9 from murine CD4+ T cells.6 It has subsequently been revealed that IFN-γ has an inhibitory effect on TH9 cell differentiation, suggesting that IFN-γ suppresses IL-9 production via STAT1 signaling as well as an indirect inhibitory effect on TH9 differentiation through the induction of IL-27 from dendritic cells.51
IL-2. IL-2 plays a prominent role in promoting IL-9 generation. It is suggested that IL-2 regulates IL-9 expression via STAT5 binding to the IL9 promoter.6,52
IL-23. Mouse studies have demonstrated that IL-23 is a negative regulator of IL-9 production.26,48
IL-25. IL-25 increases IL-9 secretion in the presence of TGF-β and IL-4 via IL-17 receptor B (IL-17RB) signaling. IL-17RB is highly expressed by TH9 cells and TH9 cells are uniquely sensitive to IL-25.53,54 IL-25 is known as a key regulator of IL-9 expression.
IL-27. Treatment of naïve CD4+ T cells with IL-27 suppresses TH9 development partially via the transcription factors STAT1 and T-bet.51
IL-1α and IL-1β. Uyttenhove et al. indicated that IL-1α and IL-1β-deficient mice have diminished IL-9 production, suggesting that IL-1α and IL-1β have a positive regulatory role on IL-9 production.55
TSLP. Thymic stromal lymphopoietin (TSLP) is an epithelial cell-derived cytokine that acts on receptors TSLPR and IL-7Rα. The addition of TSLP to primary cell cultures leads to enhancement of IL-9 production by TH9 cells in both human and mouse studies of allergic airway inflammation. Moreover, the results of this study showed that TSLP can directly promote TH9 cell differentiation and function.56,57
Activin A. Activin A is a member of the TGF-β superfamily that may also promote the differentiation of TH9 cells, yet Jones et al. reported that Activin A has a redundant function with TGF-β to induce the development of TH9 cells in both in vitro and in vivo conditions.57,58
Co-stimulatory factorsTL1A. TL1A, known as tumor necrosis factor superfamily 15 (TNFSF15), co-stimulates T cells and ILC2 via its specific receptor DR3 (TNFRSF25). In a recent murine study, it has been reported that TL1A specifically increases the differentiation of TH9 cells and induces secretion of IL-9 by signaling through DR3 associated with IL-2 and intracellular STAT5.57,59
OX40. Xiao et al. showed that OX40 (CD134), a T cell – costimulatory molecule of the TNF receptor superfamily, is a potent inducer of TH9 cells. OX40–OX40L interaction causes a prominent increase of IL-9 production through the activation of non-canonical NF-kB pathways in vitro.
This in vivo effect of OX40-induced IL-9 was seen in mouse models treated with an OX40 agonist, which then developed allergic airway inflammation characterized by eosinophil inflammation and goblet cell metaplasia.60,61
GITR. GITR (glucocorticoid-induced TNFR), which is also known as TNFRSF18, is a co-stimulatory molecule of the TNF receptor superfamily.62,63 Some studies have been shown that GITR induces IL-9 from Treg cells.25,64 Also, in a recent study, it has been reported that GITR ligation considerably increases TH9 differentiation.65
Notch pathways. Mammalian Notch receptors are classified as Notch1 to Notch4 and are expressed on all CD4+ T cells. Notch ligands can be categorized into two distinct families; the Delta like-ligands (consisting of DLL1, DLL3, and DLL4), and the Jagged ligands (Jagged1 and Jagged2).66 Conditional ablation of Notch1 and Notch2 receptors inhibits the development of TH9 cells and decreases IL-9 production in TH9 cultures. Elyaman et al. reported that Notch signaling, induced by Jagged2 ligation but not Delta-like 1, provokes TH9 cell differentiation by directly activating the transcription of IL-9 under TGF-β-based polarizing conditions. Furthermore, CD4+ T cells, which are induced by Jagged2 could produce IL-9 and play pro- or anti-inflammatory roles in amplification or inhibition of experimental autoimmune encephalomyelitis (EAE) depending on the timing of administration.67
PDL2. The other co-stimulatory factor that is known as a negative regulator of TH9 cell differentiation is programmed cell death ligand (PD-L) 2, which is a member of the B7 family. Experiments have been demonstrated that the co-stimulatory molecule PD-L2 directly and indirectly affects TH9 cell differentiation.68
COX-2. Cyclooxygenase (COX) enzymes are known to be regulators of TH1, TH2, and TH17 cells in allergic disease. It has been identified that COX-2 negatively regulates TH9 differentiation and function through an autocrine secretion of eicosanoids that cause suppression of IL-17RB.69
Signaling proteins and transcription factorsPU.1. The expression of PU.1, a key transcription factor in TH9 development, is induced by TGF-β signaling. PU.1 is expressed at higher levels in TH9 cells compared with TH1, TH2, or TH17 cells. Maturation-related histone modification in PU.1 promoter causes upregulation of the IL9 promoter enhancing TH9 development and IL-9 production.70,71 Etv5, a member of ETS family, functions in parallel with PU.1 to promote TH9 development.72
STAT6, GATA3 and IRF4. TH9 development is dependent on the transcription factor STAT6, activated by IL-4. STAT6 directly binds to the IL9 locus and plays a prominent role in directing naïve CD4+ T cells to a TH9 phenotype. GATA3 is a target gene of STAT6 and the master regulator of TH2. The transcription factor GATA3 is required for in vivo TH9 development, but is rarely expressed in in vitro TH9 cultures.4,73,74 Another STAT6 target gene, IRF4, is required for TH9 differentiation, suggesting that a deficiency in IRF4 may block the differentiation of naïve CD4+ T cells to TH9 cells, as well as controlling IL-9 secretion from TH9 cells by binding to the IL9 promoter.75
Id3, E2A and GATA3. Id3 is a transcription factor that inhibits the interaction of basic helix-loop-helix proteins, such as E2A, with DNA.76 Reduced expression of Id3 increases the binding of E2A and GATA3 to the IL9 promoter region, which promotes IL9 transcription, implicating Id3 as a potential suppressor of TH9 differentiation.77
IL-2, Jack3 and STAT5. STAT5 is a common transcription factor for multiple ligands to induce IL-9 production, and it has been indicated that the IL-2-JAK-STAT5 signaling pathway is crucial for TH9 development.6,78STAT5 knockout mice T cells have reduced IL-9-producing cells despite Th9 polarization and also a diminished ability of IL-2 to induce IL9 mRNA expression. Thus, JAK3 and STAT5 are essential for IL-2-induced IL9 expression during TH9 differentiation.52
BCL6. The master transcription factor B cell lymphoma 6 (BCL6) competes with STAT5 to bind to the IL9 promoter in T follicular helper cells with BCL6 expression reducing TH9 cell phenotype development.52 Bassil et al. also indicated that BCL6 is a negative regulator of IL-9 expression in TH9 cells and controls TH9 cell differentiation by direct binding to the IL9 locus. They also observed that IL-2/JAK3/STAT5 inhibition increases BCL6 expression and suppresses TH9 cell differentiation.79 Thus, BCL6 is a negative regulatory transcription factor for TH9 cells.
NF-κB and NFAT. Nuclear factors of activated T cells (NFATs) are a transcription factor family associated with Ca2+ dependent function. NFAT proteins control gene expression and have diverse roles in T cell subsets.80 Administration of cyclosporine A, a calcineurin inhibitor that blocks NFAT nuclear translocation to mice suppresses IL-9 synthesis, and in humans T cells NFAT expression had a negative correlation with IL-9 production.23,81–83
The NF-κB/Rel family of transcription factors, which regulate expression of many genes, bind to the IL9 promoter and facilitate transcription of IL-9.34 It has been recently shown that NFAT1 and NF-κB (p65) function synergistically for the expression of IL-9 by TH9 cells.82
Notch, Smad, and RBP-Jκ. The Smads are protein families that function as signaling intermediates for the transforming growth factor β (TGF-β) superfamily.84 This family is composed of three structurally similar proteins: Smad2, Smad3 and Smad4. Ligation of the TGF-β receptor causes phosphorylation which activates Smad2 and Smad3, which then heterodimerize with Smad4.85 Elyaman et al. proved that the activation of the Notch pathway leads to the release of the Notch 1 intracellular domain (NICD1), which recruits Smad3 and acts as a cofactor with recombining binding protein (RBP)-Jκ to bind to the IL9 promoter and increasing IL-9 production in TH9 cultures.67,86 Furthermore, Smad2 and Smad4 are required for TH9 differentiation through their actions regulating histone modifications at the IL9 locus. These data propose that the TGF-β–Smad2/4 – signaling pathway regulates IL-9 production via the epigenetic mechanism of histone modification.87
BATF. BATF (B cell activating transcription factor-like) is a basic leucine zipper protein that originates from the AP-1/ATF superfamily of transcription factors.88,89 BATF, through collaboration with IRF4, binds to the IL9 locus and activating it, promotes TH9 development as well as being required for IL-9 production in both human and mouse TH9 cells. BATF expression is dependent on STAT6, and therefore BATF may be a primary target for STAT6 within TH9 cells for IL-9 production.57,88,89
IRF1-IL-1β-STAT1. Interleukin1β induces phosphorylation of STAT1 and subsequent IRF1 expression. IRF1 binds to IL9 and IL21 promoters and enhances translation.90SIRT1-mTOR-HIF1a-glycolysis. Sirtuin 1 (SIRT1) is the highly conserved mammalian NAD+-dependent histone deacetylase which is a cellular metabolic sensor throughout several tissues.91 SIRT1 targets the IL9 gene locus and controls IL-9 production in human CD4+ T cells through SIRT1-mTOR-HIF1a-glycolysis pathway.92
Itk. Itk is a member of the Tec family of cytosolic tyrosine kinases and a mediator of T cell receptor signaling which is required for modulating T cell development and T helper cell differentiation.93 Gomez-Rodriguez et al. found a dramatic decrease of IL-9 production in Itk-deficient mice. Similarly, they showed that the Itk inhibitor reduces IL-9 expression in healthy human T cells via a dose-dependent manner. Therefore, Itk is a positive regulator of IL-9 expression and TH9 differentiation through TCR-mediated induction of IL-2 and IRF4 in both mouse and human T cells.94
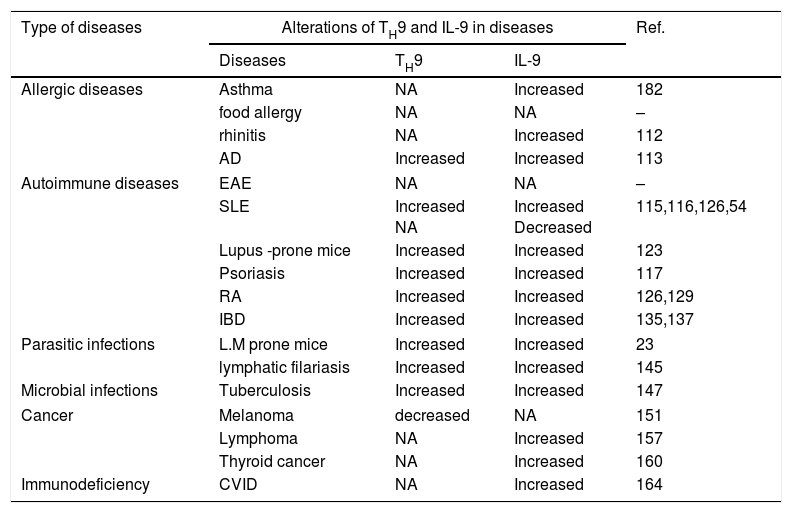
Role of TH9 cells and IL-9 in diseasesIn recent years the various functions of TH9 cells and IL-9 during immune responses have been elucidated and suggest that this cell type and cytokine have important roles in the pathogenesis of immunological diseases (Table 2). We shall discuss their roles in various immunological disorders in the following sections.
Role of TH9 and IL-9 in immunological diseases.
| Type of diseases | Alterations of TH9 and IL-9 in diseases | Ref. | ||
|---|---|---|---|---|
| Diseases | TH9 | IL-9 | ||
| Allergic diseases | Asthma | NA | Increased | 182 |
| food allergy | NA | NA | – | |
| rhinitis | NA | Increased | 112 | |
| AD | Increased | Increased | 113 | |
| Autoimmune diseases | EAE | NA | NA | – |
| SLE | Increased NA | Increased Decreased | 115,116,126,54 | |
| Lupus -prone mice | Increased | Increased | 123 | |
| Psoriasis | Increased | Increased | 117 | |
| RA | Increased | Increased | 126,129 | |
| IBD | Increased | Increased | 135,137 | |
| Parasitic infections | L.M prone mice | Increased | Increased | 23 |
| lymphatic filariasis | Increased | Increased | 145 | |
| Microbial infections | Tuberculosis | Increased | Increased | 147 |
| Cancer | Melanoma | decreased | NA | 151 |
| Lymphoma | NA | Increased | 157 | |
| Thyroid cancer | NA | Increased | 160 | |
| Immunodeficiency | CVID | NA | Increased | 164 |
Abbreviations: AD: atopic dermatitis; EAE: experimental autoimmune encephalomyelitis; SLE: systemic lupus erythematosus; RA: rheumatoid arthritis; IBD: inflammatory bowel disease; NA: not assessed.
Asthma is a chronic inflammatory disease associated with airway hyper-reactivity which has a heterogeneous phenotype. There are different subtypes of asthma; (1) allergic asthma, which is related to TH2 cell activity and its secreted cytokines, (2) non-allergic asthma (involvement of non-TH2 cell and different cells of the immune system such as ILCs), and (3) intrinsic asthma.95 Allergen specific TH2 cells and other T cells are recruited into the lung after exposure to allergens and produce a vast variety of cytokines in allergic-asthma, including IL-13, IL-4, IL-5, and IL-9. In addition, there are other subsets of TH cells, including TH9 that are involved in the pathogenesis of asthma. After IL-18 exposure, TH1 and TH9 cells contribute to the development of allergic asthma and airway hyper-reactivity through IL-18, IL-13, and IL-9 production.38,39 Non-cytotoxic ILCs are divided into three broad subtypes (ILC1, ILC2, and ILC3) and are involved across multiple functions of the immune system. Among ILCs, ILC2s are expanded by IL-25 and IL-33 and secret IL-13, IL-5, and IL-9 are believed to be involved in the pathogenesis of asthma.96,97
Intra-tracheal administration of IL-9 to mice causes an asthma-like response with induction of lung eosinophilia, increased serum total IgE levels and airway hyper-reactivity.98,99 In contrast, the blockade of IL-9 leads to a reduction of serum IgE level, lung eosinophilia, airway epithelial damage, and airway hyper-reactivity in mouse asthma model.7,100,101 In an experimental study in the lungs of transgenic mice, increased IL-9 expression resulted in airway inflammation, infiltration of eosinophils and lymphocytes, hypertrophy of epithelial cells, increased mucus generation and enhanced sub-epithelial deposition of collagen.102 Furthermore, generation of mucus and chemokine in lung epithelial cells and expression of IL-5 receptor alpha-chain on eosinophils and airway smooth muscle cells are induced by IL-9.99,103–105 The airway smooth muscle cells, isolated from human trachealis muscle, upregulated production of chemokines in response to IL-9 exposure.106 IL-9 may also be involved in the development of asthma through interaction with immune cells and in histamine-free transgenic mice, it has been demonstrated that IL-9 increases the generation of multiple cytokines from activated mast cells, including IL-1β, IL-5, IL-6, IL-10, IL-13,37 and stem cell factor (SCF).107 IL-9 can further drive AHR by up-regulating high-affinity IgE receptors on mast cells.33 Mast cells participate in acute and chronic inflammation in an asthma murine model, and increase mucus secretion, mucosal edema and bronchoconstriction in the acute phase, as well as stimulation of angiogenesis and tissue remodeling in chronic phase.33,108 IL-9 can affect naïve B cells and can promote activation of the B1 cell subset.109 IL-4, which is also increased via IL-9, can enhance production of IgE and IgG by human and murine B cells in vitro further perpetuating inflammation in AHR.24,110
Other allergic diseasesThe role of TH9 and IL-9 in several allergic diseases, including food allergy, rhinitis and atopic dermatitis (AD) have been investigated, and it has been reported that IL-9 is probably released by TH2 and TH9 cells during food allergy and the IgE-mediated acute anaphylaxis requires IL-9/IL-9R signaling and leads to mastocytosis.111 Serum levels of IL-9 have a moderate positive correlation with symptom severity including itching, sneezing, and rhinorrhea in patients with allergic rhinitis suggesting a role of IL-9 in acute allergic reactions.112
The involvement of TH9 cells and IL-9 in atopic dermatitis (AD) was highlighted when Ma et al. identified increased serum levels of IL-9, enhanced percentage of TH9 cells and up-regulated expression of the PU.1 transcription factor in AD patients compared with a control group.113 However, further studies are needed to clarify the exact role of TH9 and IL-9 in AD.
Autoimmune diseasesThe origin of the autoimmune disease is a loss of immune tolerance against self-antigens. The etiology for autoimmunity is believed to be those genetically susceptible who may be exposed to disease triggering environmental factors.114 Several studies have demonstrated that CD4+ T cell subsets such as TH1 and TH17 cells are involved in the pathogenesis of autoimmune diseases through IFN-γ and IL-17 secretion respectively. In addition, the role of TH9 cell and IL-9 in the pathogenesis of the autoimmune diseases, systemic lupus erythematosus (SLE),115,116 psoriasis,117 systemic sclerosis (SSc),115 and experimental autoimmune encephalitis (EAE)51 have been reported.
Experimental autoimmune encephalomyelitis (EAE)EAE is an experimental murine model of multiple sclerosis (MS) that is dependent on the presence of auto-reactive T cells causing central nervous system (CNS) inflammation and demyelination, which occurs due to the actins of TH cells and myelin antigens, specially PLP184–209 (myelin proteolipid protein).118
Jäger et al. demonstrated that MOG-specific TH1, TH17, and TH9 cells induce EAE after adoptive transfer and differential involvement of TH17, TH1, and TH9 cells had a similar clinical severities, but different pathological phenotypes.48 Involvement of peripheral nervous system (PNS) has not been observed in TH1 and TH17 cell mediated EAE, but inflammatory plaque was found in the PNS inTH9 cell mediated EAE.4,48 Moreover, it has been demonstrated that autoreactive TH9 cell could be involved in development of axon degeneration by Wallerian degeneration process.119 Furthermore, it has been also indicated that TH9 cell recipients have lower infiltrates of small lymphocytes in the meninges than other groups of TH1 and TH17 recipients that developed EAE. These data illustrate that mechanism of EAE induction by Th9 cells differs from the mechanisms of Th1 and Th17 cells.48
In EAE models, TH9 cells have been observed to produce IL-10 and an abundant amount of IFN-γ.48,51 IL-9 and TGF-β stimulate TH cell differentiation into TH17 cells and this effector TH17 can generate IL-9.26 Hence, this cycle deteriorates the proceeding of the disease by IL-9-induced enhancement of TH17 immune response that aggravates EAE. Li et al. demonstrated that the blocking of IL-9 using anti-IL-9 mAb leads to prevention of EAE development, reduced CNS inflammation and demyelination, decreased mRNA expression of IL-17, IL-6, IFN-γ and TNF-α in the CNS, low serum IL-17 levels, reduced CNS-infiltrated cells, diminished production of MOG-specific T cells in vivo and restriction of TH17 differentiation in vitro.70,120 Chemokines recruit pro-inflammatory cells that are involved in the pathogenesis of autoimmune disease, and in EAE, CXCR3 and CCR6 recruit TH9 cells into the CNS.121 IL-9 also influences the migration of TH17 into the CNS by upregulating the expression of CCL20 from astrocytes. Astrocytes express the IL-9 receptor complex, IL-9Rα and IL-2Rγ; and the administration of anti-IL-9 antibody diminishes EAE through the reduction of infiltrating TH17 cells and CCL20 expression by astrocytes.122
Systemic lupus erythematosus (SLE)Systemic lupus erythematosus (SLE) is an autoimmune disease that can affect the skin, joints, kidneys, brain, and other organs. SLE is an autoantibody-mediated immune complex driven disease.123,124 TH cells (especially TH17) contribute to the pathogenesis of SLE with production of inflammatory cytokines such as IL-17 perpetuating disease.123,125 TH17 cells can produce IL-9, which has a positive effect on IL-17 generation, suggesting that maybe IL-9 is indirectly involved in the pathogenesis of SLE. Yanaba et al. identified increased serum concentrations of IL-9 in SLE patients compared with healthy controls,115,116 and Ouyang et al. reported that IL-9 serum levels, mRNA transcripts, and TH9 cell percentage, were increased in SLE patients.116 It has been reported that the SLE Disease Activity Index (SLEDAI) correlates with serum IL-9 and the frequency of TH9 cells, and successfully treated patients with methylprednisolone show a reduction percentage of TH9 cells and IL-9 levels in serum.116 However, Dantas et al. found that, although there was an increase in the level of IL-9 in the serum of SLE patients compared with healthy individuals, there was no correlation between IL-9 levels and SLEDAI.126
In a mouse model of SLE, IL-9+ lymphocytes are elevated in the spleens and kidneys with increased percentages of TH9 cells and IL-9 levels in serum with positive correlation with double-stranded DNA (dsDNA) antibody titer. IL-9 blockade in MRL/lpr mice also declined serum anti-dsDNA-antibody titers and attenuated lupus nephritis.123
In contrast to these results, some studies have demonstrated that IL-9 could play a protective role in SLE disease. Pan et al. reported that IL-9 level in the serum samples of SLE patients was declined compared with healthy controls and this reduction was associated with increased oral ulcers and renal involvement, and higher anti-dsDNA and SSB autoantibodies titers.54 Furthermore, a reverse association of IL-9 with IL-23 has been reported to preserve SLE.54 IL-23, as a negative regulator of IL-9, is produced by TH17 and has an increase in SLE disease.127 In this context, Elyaman et al. indicated that the IL-9 levels are elevated in IL-23R−/− mice.26 They reported that TH17 cells produce IL-23 during a secondary stimulation and this secreted cytokine could decline the generation of IL-9.26 The discrepancy between these studies may be explained by the differences in study populations, disease activity, medication, the sensitivity of the applied assays and samples. Further investigations are essential due to these conflicting results, especially in human cases to clarify the exact role of TH9 cell – derived IL-9 in the pathogenesis of SLE.
Rheumatoid arthritis (RA)Rheumatoid arthritis (RA) is a chronic autoimmune disease characterized by inflammation and destruction of joints and the presence of auto-reactive lymphocytes and their inflammatory cytokines. Among TH cells, TH1 and TH17 cells play prominent roles in the pathogenesis of RA.128 Recently, it has been demonstrated that TH9 might be involved in the pathogenesis of RA with serum levels of IL-9 being increased in RA patients compared with healthy controls.126 IL-9 and TH9 cells are increased in RA synovial tissues and with a positive correlation between the degree of ectopic lymphoid follicle organization and IL-9 expression.129 It has also been revealed that the expression of both the mRNA and protein of IL-9R, IL-4, TSLP and TGF-β are elevated in RA synovial tissue, indicating a possible role of TH9 and IL-9 in the development of RA.
PsoriasisPsoriasis is characterized as a chronic inflammatory skin disease that is associated with infiltration of inflammatory cells such as T cells, macrophages, and mast cells, as well as enhancement of angiogenesis through the dominant presence of TH1/TH17cells. Singh et al. investigated the role of TH9 and IL-9 in K5.hTGF-b1 transgenic mice with a similar phenotype to human psoriasis and indicated a link between IL-9 and TH17 pathway within the disease.130 They also identified an elevated expression of IL-9R and IL-9 in affected skin and demonstrated that intradermal IL-9 injection could induce TH17-related inflammation. IL-9 not only induces angiogenesis and overexpression of VEGF and CD31 in mice in vivo but also elevates tube creation of human endothelial cells in vitro. Administration of anti-IL-9 antibody in this mouse model of psoriasis reduced inflammation, angiogenesis and delayed the onset of the psoriatic skin phenotype. Human studies of psoriasis have reported increased TH9 cells in skin lesions of patients.131,132 According to these findings, it seems that TH9 cells have a potential role in the pathogenesis of psoriasis by the production of IL-9 and influence TH17 cell-related cytokine production.
Inflammatory bowel diseaseCrohn's disease and ulcerative colitis are the most prominent forms of inflammatory bowel disease (IBD). These disorders occur in the gastrointestinal tract with chronic inflammation in the absence of a recognized pathogenic infection. TH1 is the dominant subset of TH cells in Crohn's disease, which secretes TNFα and IFNγ. TH2 cells are believed to play a dominant role in the pathogenesis of ulcerative colitis via generation of cytokines including IL-5 and IL-13.133 An imbalanced TH17/Treg cell presents in both diseases leads to overexpression of TH17 cells and a decline of Treg cells.134
TH9 cells are increased in the gastrointestinal tract of patients with both Crohn's disease and ulcerative colitis in relapsing and remission phases compared with healthy control.135–137 It has been reported that the transfer of both CD4+CD45RBhigh naïve T cells and TH9 cells into RAG-deficient mice exert enhanced development of ulcerative colitis compared with adoptive transfer of naïve CD4+CD45RBhigh T cells into these mice.4 A deficient-PU.1, lacking TH9 cells, mouse shows reduced colitis.137
Regarding the role of IL-9, the secretion of IL-9 by TH9 is necessary for the existence of colitis. Blocking IL-9 by anti-IL-9 antibody in mice, and also in those mice lacking PU.1 expression in T cells and therefore TH9 cells, resulted in the remission of colitis.137 Moreover, it has been reported that IL-9 production by TH9 cell is only found in colitis and there is a correlation between IL-9 expression, the number of TH9 cells and severity of colitis. In ulcerative colitis, it has been observed that IL-9R expression is enhanced in epithelial cells,135,137 and that IL-9 can also contribute to the development of inflammatory colitis via GI mast cell activation.138
Gerlach et al. reported a regulatory role for TH9 cells in the experimental TNBS (2,4,6-trinitrobenzene sulfonic acid) colitis model, with the deficiency of IL-9 suppressing TNBS-induced colitis with lower numbers of PU.1 expressing T cells in the lamina propria.138 It has been identified that at the tight junction, the protein Claudin1 had lower expression levels when IL-9 was absent, while the pore-forming molecule Claudin2 demonstrated equal expression in TNBS-treated wild-type and IL-9-deficient animals, suggesting that IL-9 can regulate intestinal permeability via its effects on tight junction molecules. Therefore, modulation of IL-9 function emerges as a new approach for regulating barrier function of it in intestinal inflammatory diseases such as IBD.138
Infectious diseaseParasitic infectionsIn parasitic infections IL-9 and TH9 cells play a protective role. IL-9 has an important role in the generation of mucus and enhancement of intestinal permeability leading to diarrhea in GI parasitic infections.7 In a study, BALB/c mice, which are a murine model susceptible to Leishmania major, were infected by Leishmania major (L. major), then levels of IL-9 were evaluated. Lymphocytes in lymph nodes and spleens of L. major-infected BALB/c mice produced higher levels of IL-9 during an acute immune response compared with C57BL/6mice.23 Licona-Limón et al. also demonstrated a prominent role for IL-9 against Nippostrongylus brasIliensis in mouse models infection.139 They report that although TH9 cells and ILC2s were major sources of infection-induced IL-9 production, the adoptive transfer of TH9 cells, but not TH2 cells, led to rapid worm expulsion, significant basophilia, and elevated mast cell counts in RAG2-deficient hosts, indicating a critical and non-redundant role of TH9 cells and IL-9 in host-protective type 2 immunity against parasitic worm infection.139 Another up-regulatory role of IL-9 was described by Turner et al.: IL-9 acts as an autocrine amplifier of ILC2 and leads to enhancement of tissue repair in the recovery phase of helminth-induced lung inflammation.140
In humans Pang et al. showed the involvement of TH9 cells and IL-9 in the immune response against Echinococcus granulosus infection.141 Muscle hyper-contractility, which helps to eliminate helminth infection, could also be under different immunological control depending on the species of nematode infection.142 In infected mice with Trichinella spiralis, IL-4 and IL-13 are sufficient for hyper-contractility, and although IL-9 can ease worm expulsion, it is not crucial. On the other hand, in mice infected with Trichuris muris, IL-9 is required for an optimal response with IL-9 accelerating worm expulsion during T. muris infection by hypercontractility of colonic muscle.142 Transgenic expression of IL-9 can exert a resistance against infection, which is associated with increased IgE and IgG1 levels and high intestinal mast cell infiltrate.143 Administration of anti-IL-9 antibody to the infected murine model with T. muris induces high susceptibility to infection due to the prevention of mast cell activation and eosinophilia.144 It has been identified that the deficiency of TGF-βR2 in mice is also associated with impaired IL-9 production, elevated worm burden and diminished mast cells in the cecum.3 It seems that decreased IL-9 or defected IL-9R and TGF-βR2 result in increased sensitivity to infections through reduction of mast cell and eosinophil activation.
In one human study, it has been demonstrated that Ag-specific TH9 cells are elevated in individuals that suffered from lymphatic filariasis and inhibition of IL-9 or its downstream targets can alleviate the filarial disease.145 Anuradha et al. examined the role of TH9 in Strongyloidis stercoralis (Ss) by comparing the frequency of TH9 cells in infected and non-infected individuals, and found increased antigen specific expression of TH9 cells in infected individuals and higher amounts of IL-9 cytokine in the supernatant of whole blood stimulated with Ss antigen.146 Furthermore, they inferred that the observed increased frequency of TH9 cells is dependent on IL-10 and TGFβ as neutralization of these cytokines decreased TH9 frequency.
Therefore, it appears that immune responses against parasitic infections with TH9 cells and IL-9 are dependent on the types of helminth infections and may play both redundant and non-redundant roles in helminth immunity.
Microbial infectionsSeveral studies have investigated the role of TH9 and IL-9 in microbial diseases such as mycobacterial and gram-negative infections. Tuberculous pleural effusions (TPE) are a severe delayed-type hypersensitivity reaction in response to the mycobacterial antigens in the pleural space, which is associated with the accumulation of lymphocytes; particularly TH cells. Both supernatants of cultured pleural mesothelial cells (PMCs) and during clinical TPE, TH9 cell recruitment is mediated by CCL20 with CCR6 in TH9 cells. Pleural mesothelial cells also stimulate TH9 cell differentiation by MHC antigen presentation. IL-9 is involved in repairing PMCs injured through Mycobacterium tuberculosis infection and inhibits apoptosis in PMCs, indicating that IL-9 is able to ameliorate wound healing and long term recovering of PMCs in vitro.45 However, it has been demonstrated that overexpression of IL-9 may influence persistence of infection with tuberculosis by suppressing TH1 cell population and its cytokines.147 In this context, Grohmann et al. reported that administration of recombinant IL-9 to infected mice with Pseudomonas aeruginosa leads to declined inflammatory cytokines, such as TNF-α, IFN-γ and IL-12 and also induces anti-inflammatory cytokines such as IL-10.148 Furthermore, an in vitro study on human monocytes has clarified that IL-9 can abate lethal shock reaction by inhibition of inflammatory cytokines generation, numerous monocyte functions, and reactive oxygen intermediates (ROI) production.149
Tumor immunityA correlation between single nucleotide polymorphisms in the IL9 gene and an enhanced risk of cutaneous malignant melanoma was first described in 2009 by Yang et al.150 In recent years, the relationship between TH9 cell, IL-9 and melanoma has been investigated and reported that TH9 cells play a potential protective role in melanoma.151 Parallel with this role of TH9 cells in melanoma, Fang et al. showed that IL-9 inhibits melanoma HTB-72 cell growth by upregulation of P21 and TRAIL.152 The administration of anti-IL-9 antibody can increase tumor growth, and progressive tumor growth is observed in IL9rR−/− murine models. TH9 cells transfer into the B16–F10 murine model reduces the severity of melanoma and tumor masses compared with the control group, and rIL-9 administration inhibits tumor growth in Rag1−/− mice, but not mast cell – deficient mice, suggesting that mast cells are an important cellular effector target of rIL-9.151 In an interesting study in this context, Abdul-Wahid et al. showed that by disturbing mast cells survival and function, via anti-CD117 antibody and cromoglycate respectively, led to abolished immunity against tumors induction in mice, highlighting the importance of mast cells in mediating TH9 cell-dependent anti-tumor immune responses and tumor growth prevention.153
The antitumor effect of TH9 cell and IL-9 has also been investigated in a lung adenocarcinoma model, with potent antitumor function of TH9 cell and IL-9 demonstrated.49 Mechanisms of anti-tumor immunity mediated by TH9 cells are via cytokine production such as IL-9 leading to the expression of CCL20 by tumor cells that causes chemoattraction and migration of immune cells including dendritic cells (DC) and CD8+ T cells by CCR6.154 In addition, IL-3 and IL-21 produced by TH9 and exert antitumor immunity via dendritic cell (DC) activation and CD8+ T cell secretion of IFN-γ.155 TH9 cells directly contribute to tumor cell death by cytotoxic release of granzyme-B.151 It has been recently described that Ag-specific TH9 cells are able to inhibit squamous cancer (SqC) growth and also promote apoptosis in SqC both in vivo and in vitro.156
However, the reverse function of IL-9 in some cancers has been reported. IL-9 can promote human malignant lymphoma and it has high expression in anaplastic lymphoma and Hodgkin disease.157 A tumor-promoting role of TH9 cells is reported in hepatocellular carcinoma via elevation of CCL20 and suppression of STAT3.158 Moreover, it has been shown that transgenic IL9 expression induces spontaneous lymphomas in a murine model.159 In new research, increased IL-9 levels were observed in differentiated thyroid cancer patients compared with control subjects,160 and IL-9 increased the rate of proliferation of tumor cells and inhibited cancer cell apoptosis.161,162 Ye et al. demonstrated that IL-9 could induce proliferation, migration and adhesion of human lung cancer cells and completely inhibit the IFN-γ-induced apoptosis of these cells.45 Chen et al. reported that addition of recombinant human IL-4 (rIL-4) to cultured human B cell lymphoma cells (MEC-1) caused increased expression of STAT6 and IL-9, and that overexpression of IL-9 promoted the pathogenesis of chronic lymphocytic leukemia.162 IL-9 may also enhance of regulatory T cell functions and restrain tumor immunity in vitro.163
Although the underlying reason for these controversial findings is not yet clear, it can be related to the distinctive expression of IL–9R or the differences in the level of IL-9 production by the cancer cells. TH9 cell and IL-9 can appear as a pro- or anti-tumorigenic agent, thus further studies are essential to clarify these different findings regarding the role of TH9 and IL-9 in tumor immunity.
Immunodeficiency disordersThere are limited studies of the role of TH9 and IL-9 in immunodeficiency diseases. In a recent study, we indicated that the patients who suffered from common variable immune deficiency (CVID) have overexpression of IL-9 mRNA level in their peripheral blood compared with healthy controls.164 CVID is a primary immunodeficiency syndrome that is associated with defective B cell and T cell abnormalities.165–167 We indicated that the level of IL-9 was higher in CVID patients who have non-infection complications like autoimmune or allergic disorders.164 Since autoimmune and allergic diseases are more prevalent in CVID patients,168,169 elevated IL-9 mRNA level may be due to these complications.164 However further studies need to be performed. Moreover, as atopic diseases are common clinical manifestations with high prevalence in patients with selective IgA deficiency (SIgAD),165,170 further investigation of IL-9 and TH9 cells in SIgAD patients are needed.
Therapeutic strategyIn some diseases, the role of TH9 cell and its cytokines such as IL-9 and IL-21 have been investigated as a therapeutic solution. For instance, recombinant IL-21 was investigated in a phase II study of patients with metastatic melanoma and a 22.5% response rate was achieved in the first-line treatment.171 As previously described, IL-21 is produced by TH9 cell; especially in the presence of IL-1β cytokine.49 TH9 cell could generate more IL-21 when IL-9 is down-regulated, yet IL-21 can affect Bcl6 expression with Bcl6 having an inhibitory role on the IL-9 generation. Thus, IL-21 is able to exert a positive feedback for its own generation.52
Administration of TH9 cells, as a therapeutic strategy, leads to stimulation of CD8+ T cells and DCs recruitment into tumor site, which mediates antitumor immunity. Direct administration of TH9 cells leads to specific homing of transferred T cells to the tumor site and more cytokine production compared with systemic injection of IL-21.172 TH9 cells promote overexpression of anti-apoptotic protein Bcl-xL and activation of p38, ERK, and STAT5 signaling pathways in DCs.155 These approaches suggest a new avenue for cancer immunotherapy, but treatment with TH9 or rIL-9 should be carefully considered depending on cancer type; particularly for lymphoma and other tumor cells, which express receptors for IL-9.151
Due to the harmful role of IL-9 in the pathogenesis of asthma and AHR, blocking strategies of IL-9 are developing for such diseases.173,174
ConclusionNaive T CD4+ cells differentiate into TH9 cells by exposing to a specific antigen and a combination of IL-4 and TGF-β. IL-9 is the signature cytokine for TH9 cell subset and the secretion of this cytokine is associated with the presence of other cytokines. There are several cytokines that can increase IL-9 secretion and TH9 cells development including IL-2, IL-25, IL-1α and IL-1β, TSLP, TL1A, and Activin A. TH9 differentiation can be inhibited by some other cytokines such as IFN-γ, IL-23, and IL-27. There are many transcription factors including PU.1, IRF4, STATs, NFAT, GATA1, GATA3, Smads and Notch, NF-κB, and AP-1, which are involved in the TH9 production. It seems that TH9 cells are pro-inflammatory cells and their exact function probably depends on the tissue microenvironment and the presence of other TH cell cytokines in the inflammatory milieu.175 TH9 cells and IL-9 play harmful roles in the pathogenesis of asthma and other allergic diseases and in autoimmune diseases TH9 cells and IL-9 have dual roles, but appear to be detrimental overall in autoimmune diseases pathogenesis. In microbial and parasite infections, TH9 cells and IL-9 have broadly protective roles, although these are often pathogen specific. TH9 cells mostly appear as anti-tumorigenic cells, but their cytokines indicate different roles in various tumors. For instance, IL-21 has a potent protective role in the melanoma, but IL-9 emerges as pro-tumorigenic in the lymphoma. Taken together, various functions and the different presence of TH9 cells and IL-9 in the mentioned diseases, show the need for more studies to approve the administration of IL-9 and TH9 cells as an immunotherapeutic strategy in different conditions.
Conflict of interestThe authors declare that they have no conflicts of interest.