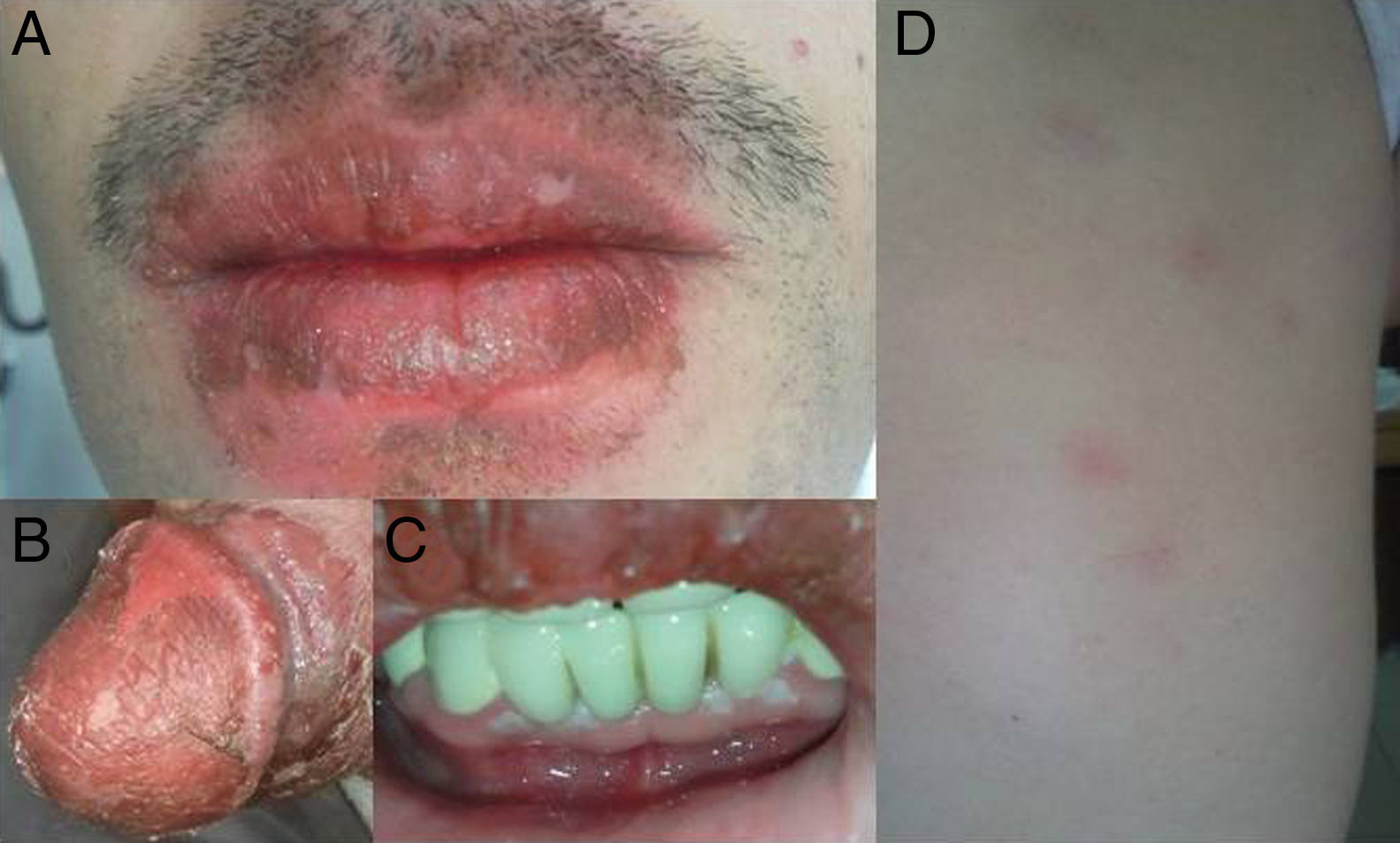
Fixed drug eruption (FDE) usually appears as a solitary/small number of pruritic, well circumscribed, erythematous–violaceous macules that evolve into oedematous plaques which typically resolve after the discontinuation of the offending drug, leaving residual hyperpigmentation. This eruption is considered pathognomonic of a drug-induced dermatosis and is triggered within a few minutes or up to several hours after medicine intake.1 Lesions re-occur on exactly the same sites when the offending drug is re-administered. FDE can occasionally present itself through a wide spectrum of clinical manifestations, but multifocal bullous FDEs are rare and require differentiation from other blistering diseases, like herpes labialis, localised bullous pemphigoid, discoid lupus erythematosus and toxic epidermal necrolysis (TEN).
Etodolac, a pyranocarboxylic acid, known as a COX-2 selective inhibitor, is a widely used analgesic-antipyretic with a consistent safety profile. Its cutaneous side effects are rare, varying from pruritus or rash to fatal TEN.2 There are only two publications so far about etodolac-induced FDE, with non-pigmenting/pigmenting limited cutaneous lesions, however, multifocal bullous FDE has not been previously described as one of them.3,4
A 30-year-old man with perennial mild allergic rhinitis was referred to our unit with a 6-month history of recurrent angio-oedema/urticaria episodes, which occurred 30min and 13h after flurbiprofen and paracetamol intake, respectively. His medical history revealed frequent non-steroidal anti-inflammatory drugs (NSAIDs) intake for his headaches and two attacks of orogenital bullous ulcerations along with erythematous eruption over his extremities in the last 12 months. Lesions were recurrent at the same locations, always occurring 5–7h after taking etodolac and then resolving in 2 weeks; and the last attack was eight months before. His family practitioner diagnosed these lesions as herpes infection, and prescribed acyclovir.
There was no other significant medical history. At the time of clinical evaluation his physical examination was normal. The patient's blood workup, including blood counts with differential, complete metabolic panels and total IgE levels, were unremarkable except his thyroid function tests. His serum concentrations of TSH, anti-thyroglobulin and anti-peroxidase were elevated, which uncovered his occult Hashimoto's thyroiditis (HT). Immediately afterwards levothyroxine therapy was initiated by the consulting endocrinologist. His skin prick testing with common aeroallergens was positive to D. pteronyssinus, D. farina, Blatella, and grasses/cereals (Allergopharma, Germany). After his hormone levels were normalised, and since he described multiple cutaneous reactions with NSAIDs, we performed single-blind, placebo-controlled oral challenge tests as the gold standard in diagnosis. Increasing doses were administered at 30-min intervals under strict hospital surveillance, until the cumulative daily therapeutic dose was reached. The challenges with flurbiprofen, paracetamol, meloxicam and nimesulid were negative.
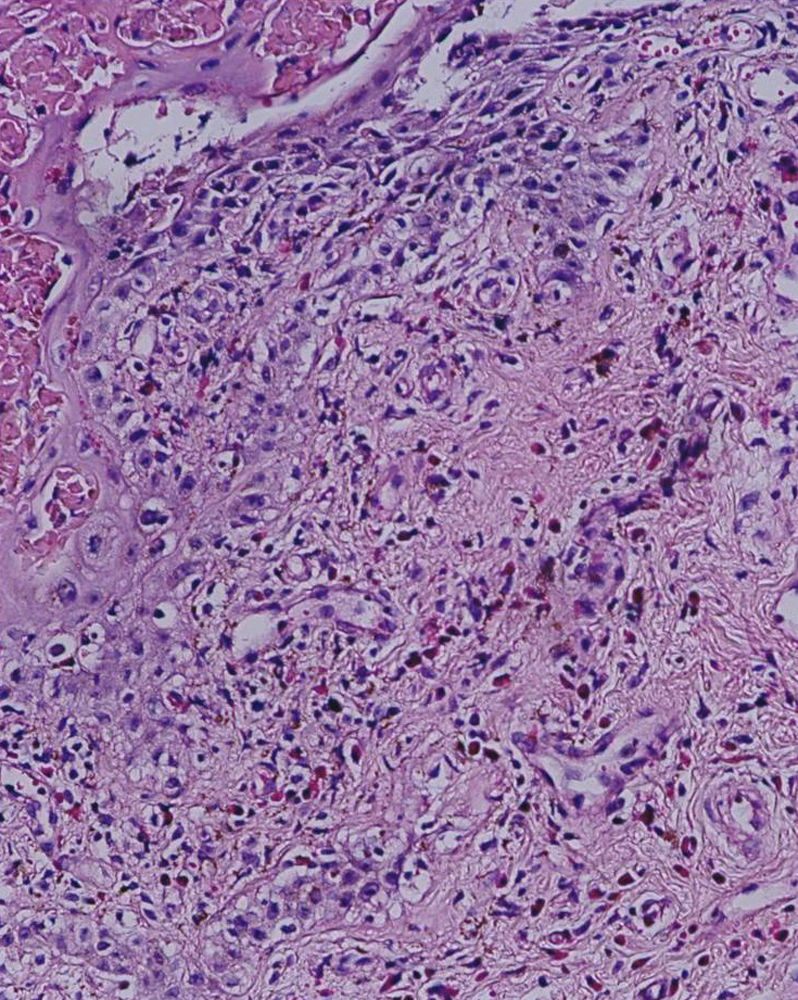
However within 6–8h of intake of etodolac (total dose: 750mg), he developed a burning sensation over oral and genital mucosa followed by erythema. The next day, pruritic, violaceous-erythematous plaques of 2–4cm in diameter appeared on his right hand, left foot and right scapula, along with multiple vesiculobullous-ulcerated lesions around his lips, penis and oral mucosa (Fig. 1). When compared with the previous two attacks, neither an increase in the number of lesions and the intensity of pruritus, nor shortening of the time interval between drug intake and appearance of symptoms was described by the patient. The differential cell counts in blister fluid revealed: 10% polymorphonucleated leukocytes, 28% monocytes, 60% lymphocytes, 2% eosinophils. The dermatopathologic examination of the biopsy taken from the subcoronal region of the penis exhibited a subepidermal blister with prominent vacuolar changes along the dermoepidermal junction, mixed with inflammatory infiltrate consisting predominately of lymphocytic and a few eosinophils around the superficial plexus (Fig. 2). Clinicopathological findings yielded the diagnosis of multifocal bullous FDE. The lesions healed within 2 weeks by using hydrocortisone 17 butyrate 0.1% fatty cream treatment, leaving slight residual pigmentation. The patient was advised not to take etodolac again, and was given written information on the alternative NSAIDs. Upon avoidance of etodolac, no other attack was observed during a 12-month follow-up.
NSAIDs-induced hypersensitivity reactions involve different mechanisms and present a wide range of clinical manifestations from anaphylaxis developing within minutes after drug ingestion to delayed-type responses appearing after days and weeks.5 Since there is a wide clinical spectrum and several various mechanisms (both immunological and non-immunological) in patients referred with a history of hypersensitivity, a proper diagnosis should be confirmed by a challenge with a culprit drug if appropriate, and safety of the alternative drug should be confirmed by oral challenge as recommended by review of the EAACI/ENDA and GA2LEN/HANNA.6
The entity of FDE was first reported by Bourns in 1889 and the name was assigned by Brocq in 1894, who described an “éruption érythémato-pigmentée fixe”.1 FDE has various morphological types of lesions, which are frequently misdiagnosed, leading to recurrent eruptions exactly on the same sites when the offending drug is re-administered, as in our case. These must be correctly recognised to indicate the appropriate therapy. The maximum form is generalised/multifocal bullous FDE, which can clinically or histologically mimic TEN. Recent studies have shown that CD8+T lymphocytes found in FDE have the same phenotypic features as those in TEN.
Although FDE may occur anywhere on the skin or mucous membrane, sites of predilection are not only the palms and soles, the medial aspects of the limbs, abdomen, but also lips, tongue, oral mucosa and in men the glans penis. The lesions are often asymptomatic, but may be accompanied by burning and pruritus at the involved sites, as seen in our patient. Rarely systemic signs and symptoms such as malaise, anorexia, fever, diarrhoea, nausea and abdominal complaints may be present. FDE can affect any age group from childhood to older age, but most commonly individuals aged 20–40 years.1,7
The fixed drug eruption is a common drug side effect constituting about 5–10% of cutaneous drug reactions. It is observed most frequently after oral administration, less frequently after intravenous or intramuscular administration or topical application. Although drugs causing FDE differ in countries depending on the availability of the various drugs, the most frequent drugs associated with FDE are antibiotics, antifungal drugs, analgesics, antiphlogistics, anticonvulsants and barbiturates.1 Even though NSAIDs are among the most widely used medications, the newer cyclo-oxygenase enzyme 2 (COX-2) inhibitors have rarely been reported to cause FDE.8
Pathogenetically, FDE represents a drug-induced non-immediate type IVc reaction. Intraepidermal interferon-γ producing αβTCR+ CD8+ T cells, possessing a phenotype resembling effector memory T cells, which are found abundant both in resting and acute lesions, play the predominant role in pathogenesis. They are primarily responsible for the epidermal damage.7 There is also evidence of the expansion of IL-10 producing CD4+ T cells, especially CD25+FoxP3+ regulatory T cells, in FDE, which may be responsible for its spontaneous resolution and may determine the different evolution and prognosis of FDE and of TEN.7,8 But the factors that determine the evolution of these two distinct conditions are unknown.
The most important measure in diagnostics is an exact drug history, as patients tend to disregard the significance of “on-and-off” medications and therefore fail to report them. Systemic provocation still represents the gold standard method for establishing the aetiological agent in FDE; however, convincing results can be also achieved with topical provocation of certain drugs when applied to sites of previous FDE lesions although a negative skin reaction gives no reliable information.
Subjects who experience an adverse reaction to a single drug sometimes display similar reactions to several others. The most common clinical manifestations of multiple drug hypersensitivity (MDH) are urticaria and/or angio-oedema, but also anaphylactic shock or FDE might occur.5 In our case, since the patient was associating his recurrent angio-oedema/urticaria episodes with NSAIDs and also since NSAIDs are the main reported group of drugs in polysensitisation reactions; we investigated the diagnosis of “NSAIDs-exacerbated urticaria/angio-oedema” using the “gold standard” oral provocation test, which yielded negative results for drug hypersensitivity.6
Thyroid autoantibodies (TA) have a prevalence of about 3–6% in the general population versus about 15–30% in chronic idiopathic urticaria (CIU) patients, suggesting that these two disorders are associated. The mechanisms for the apparent association between CIU and serological evidence of thyroid autoimmunity are not clear.9 The effects of replacement treatment for hypothyroidism on clinical symptoms of urticaria are still controversial. Leznoff et al. reported that the l-thyroxin therapy improved clinical symptoms of CIU.10 In our atopic patient, instead of NSAIDs hypersensitivity, the concurrence of HT might possibly be responsible for his urticaria/angio-oedema, which probably reflects a shared genetic predisposition toward the development of autoimmune disease. Also levothyroxine treatment had a positive influence on the clinical course of his urticaria.
This is a unique presentation of multifocal bullous FDE due to etodolac, which has not been previously described in the literature. We propose that etodolac should be added to the list of drugs causing multifocal bullous FDE, so that these lesions can be correctly recognised to indicate the appropriate therapy. This case also illustrates the importance of following the stepwise approach to the diagnosis of hypersensitivity to NSAIDs, both while taking the history as well as deciding on the implementation of diagnostic procedures.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of data. The authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestThe authors have no conflict of interests to declare.
Presented at the XXX Congress of the European Academy of Allergology and Clinical Immunology, 10–15 June 2011, Istanbul, Turkey.