Fixed drug eruption is characterised by the sudden onset of round and/or oval, oedematous, dusky-red macules on the skin and/or mucous membranes accompanied by burning and/or itching. The acute phase is usually followed by residual pigmentation.1 The incidence of fixed drug eruption induced by a specific drug appears to depend on frequency of use.2
Quinolones are generally well tolerated, and their spectrum of adverse reactions ranges from gastrointestinal symptoms, which are the most frequent, to neuropsychiatric symptoms, haematologic abnormalities, and hypersensitivity reactions.3
Norfloxacin has proven particularly useful in treating urinary tract infections. Cutaneous side effects occur in less than 1% of patients and include urticaria, rash, and pruritus.4
We report a case of norfloxacin-induced fixed drug eruption and cross-reactivity with ciprofloxacin and moxifloxacin.
A 40-year-old woman with no history of atopy or drug allergy presented three years ago with pruritic erythematous macules on the back of her left wrist a few hours after taking norfloxacin (Normon SA, Madrid, Spain). The skin lesion resolved some days later without treatment, leaving a hyperpigmented lesion measuring 3cm in diameter.
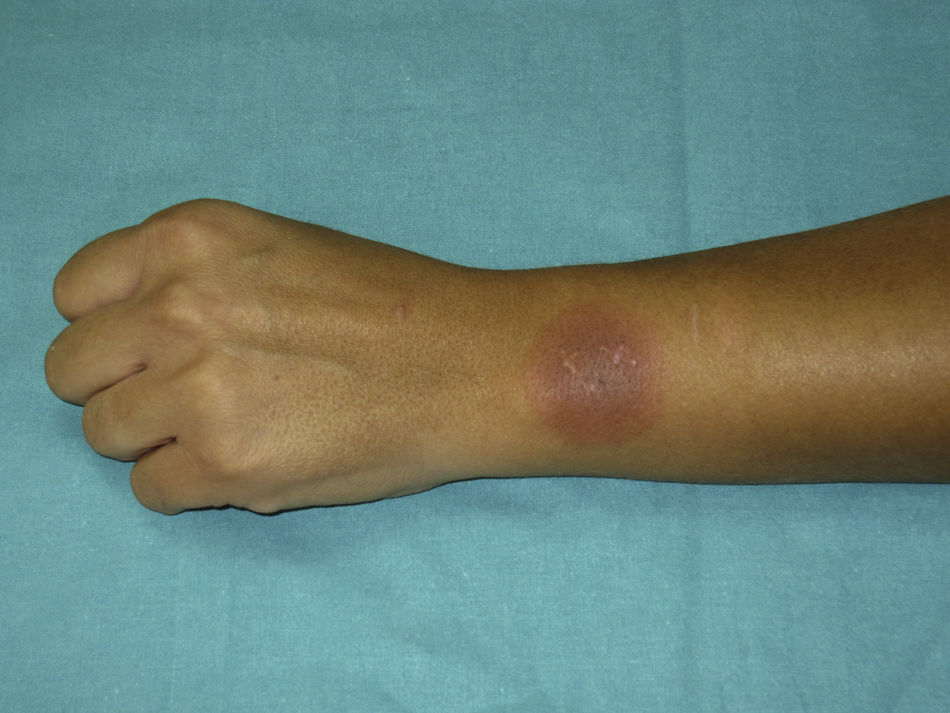
Six months ago she was treated with norfloxacin (Normon SA, Madrid, Spain) for a urinary tract infection. Five hours after ingestion of the first tablet, she developed pruritus on her upper lip and on the back of her left wrist. Twenty-four hours after ingestion, she presented a bullous lesion on her upper lip and an erythematous macule measuring 5cm in diameter on the residual lesion of the left wrist. Biopsy of the wrist lesion revealed a lichenoid infiltrate of lymphocytes with prominent vacuolar degeneration and melanin incontinence consistent with fixed drug eruption. The lesions subsided without treatment after approximately 48h.
After obtaining the patient's informed consent, we performed skin prick tests with norfloxacin (1mg suspended in 1ml saline), moxifloxacin (4mg/ml), ciprofloxacin (1mg/ml), and levofloxacin (5mg/ml). The results were negative. Histamine (10mg/ml solution) and buffered saline were used as positive and negative controls, respectively. We also performed intradermal tests with norfloxacin (0.001 and 0.01mg/ml dilution), moxifloxacin (0.004mg/ml dilution), ciprofloxacin (0.001 and 0.01mg/ml dilution), and levofloxacin (0.005 and 0.05mg/ml dilution), all with negative results.
Patch tests were applied on the upper back with norfloxacin, moxifloxacin, ciprofloxacin, and levofloxacin and on the wrist lesion with norfloxacin, all at a concentration of 20% in petrolatum. Patch tests (Nonweven Patch Test Strips Curatest®; Lohman & Rauscher International, Rangsdorf, Germany) were read at 48 and 96h, with negative results. A lymphoblastic transformation test with norfloxacin, moxifloxacin, and levofloxacin at 10 and 1mg/ml also gave negative results.
Therefore, we performed a single-blind oral challenge with norfloxacin. The result was positive with a delayed reaction. Oral challenge testing was performed with norfloxacin (125, 250, and 500mg) at gradually increasing doses at 1-h intervals. Twenty-four hours after the oral challenge with norfloxacin (cumulative dose of 875mg), the patient developed pruritus and erythema on the wrist lesion and a bullous lesion on her upper lip (Fig. 1).
To investigate possible cross-reactivity between quinolones, we performed an oral challenge with levofloxacin and moxifloxacin. The patient developed a bullous lesion on her lip and erythema on the wrist lesion with both drugs. Finally, the patient was advised to avoid quinolones.
Quinolones can induce both IgE-mediated hypersensitivity and delayed reactions such as fixed exanthema.5
The exact pathogenesis of fixed drug eruption is unknown, although antibodies, serum factors, and cell-mediated immunity have been implicated. Antibody-dependent cell-mediated cytotoxicity may also play a part in its pathogenesis.6
Patch testing is a simple and safe method to identify specific causative agents of fixed drug eruption, although it cannot be considered reliable if the results are negative.7 In our case, we performed the patch test on normal skin with norfloxacin, moxifloxacin, ciprofloxacin, and levofloxacin and on previously affected skin with norfloxacin, all in 20% petrolatum jelly. The results were negative in all cases. Previous reports have shown conflicting patch tests results.2,5,8 The election of an inadequate vehicle or alteration of the molecular structure of the drug may be implicated. However, we believe that challenge testing is the gold standard for the diagnosis of this adverse reaction.
Our literature review revealed two reports of fixed drug eruption due to norfloxacin. Fernández-Rivas8 described a patient with several episodes of fixed drug eruption after ingestion of norfloxacin. Patch tests were negative with all the quinolones tested and the patient tolerated ciprofloxacin. In contrast with that case, our patient did not tolerate the other quinolones tested.
Rodríguez-Morales et al.9 reported a case of fixed drug eruption from quinolones (ciprofloxacin and norfloxacin) with a positive result in the patch test (ciprofloxacin) applied to the lesion. All patch tests were negative in our patient.
Controlled oral challenge seems to be the only way to determine the diagnosis and to demonstrate cross-reactivity, since the results of patch testing are inconclusive.5
In summary, we present a case of fixed drug eruption induced by norfloxacin, as confirmed by oral challenge and biopsy of the lesion.
Conflict of interestThe authors have no conflict of interest to declare.
This work was supported by grants from Spanish Health Ministry (FIS) network RIRAAF (RD07/0064).