Hypersensitivity pneumonitis (HP) is a pulmonary disease with symptoms of dyspnoea and cough resulting from the inhalation of an antigen to which the patient has been previously sensitised.1
HP is a disease that occurs as a consequence of exposure to organic dust. Initially, it was associated with farming (mouldy grain or hay handling), hence the term farmer's lung.2 With time, a large variety of environmental settings and antigens have been described.3
HP caused by inhalation of mushroom spores has increased, and HP has been described as an occupational hazard of mushroom plant workers.4
Edible oyster mushrooms, Pleurotus species, are cultivated all over the world.5
The indoor cultivation of Pleurotus osteatus regularly led to allergic symptoms in workers.6
IL-22 is a pro-inflammatory cytokine belonging to the IL-10 family and represents an important effector molecule of activated lymphocyte T helper (Th)22, Th1, Th17 cells.7
IL-22 increases the innate immunity of tissue cells, protects tissues from damage and enhances their regeneration. It exerts pro-inflammatory effects, is present in systemic and local inflammation, and exerts an important role in the antimicrobial defence.8
Given the fact that IL-22 prominently increases the expression of a range of antimicrobially acting proteins in various epithelia suggests a role for this cytokine in the innate immune defence, especially against extracellular bacteria. Moreover, further mechanisms used by IL-22 to promote the defence against intracellular bacteria, fungi, viruses and parasites are currently under exploration.9
Simonian et al. showed that a subset of γδ T cells represents the predominant source of Th17 cytokine IL-22 in a murine model of HP. Preventing expression of IL-22 they accelerated lung fibrosis. Direct blockage of IL-22 also enhanced collagen deposition in the lung, whereas administration of recombinant IL-22 inhibited lung fibrosis. These data revealed a protective pathway that involves the inhibition of γδ T cells by regulatory IL-22-secreting γδ T cells.10
Therefore we evaluated IL-22 serum levels in a patient suffering from HP and then we compared them with seven healthy subjects of the same sex and similar age (mean±SD: 9.45±6.14pg/ml).
IL-22 serum concentrations were measured by a quantitative enzyme immunoassay technique. The assay was performed using a commercially available kit (R&D Systems Europe Abingdon, UK).
A 23-year-old man reported recurrent episodes of chest tightness, cough, wheezing symptoms accompanied by rhinoconjunctivitis, fever (37.5–38.5°C), and fatigue; these symptoms occurred in the evenings after work for about a year.
The patient reported to work continuously for about three years in a greenhouse, where he cultivated edible mushrooms (P. osteatus); moreover he stated that his contact, even if occasionally, had began about ten years before.
We carried out the common blood tests; the differential blood count showed a slight increase in white blood cells (11,660mm3) and decrease in haematocrit (37.5%), the C-reactive protein (CRP) and the fibrinogen were over the range (14.60mg/dl and 410g/dl respectively) while serum iron was below the standard (25mcg/dl), the specific IgE were positive for Dermatophagoides farinae (18.90KU/l), Dermatophagoides pteronyssinus (21.00KU/l), Cynodon dactylon (2.33KU/l), Fetuca elaitor (6.46KU/l), Cladosporium herbarum (6.21KU/l), and Parietaria judaica (1.13KU/l).
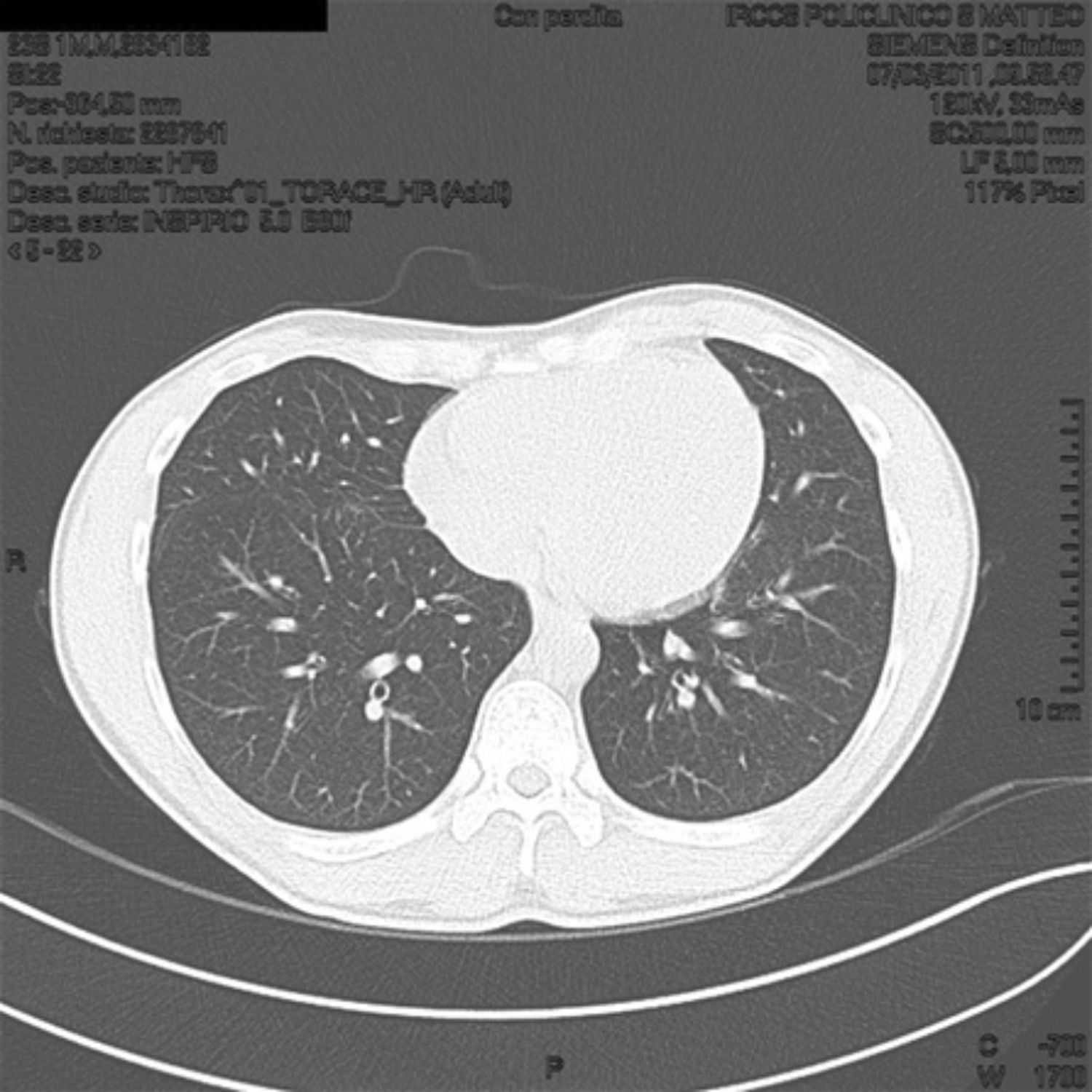
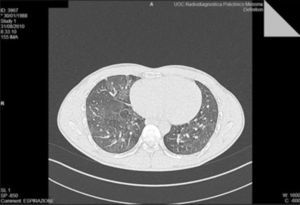
Positive specific IgE could explain the respiratory symptoms but not the fever, so we performed a chest X-ray that showed striated patchy opacities; proceeding in the diagnostic work we performed a high resolution computed tomography (HRCT) lung scanning that showed in the expiratory phase a marked and uneven alteration in density in both lungs for the coexistence of higher density and hyperlucency areas (part of the mosaic attenuation). These areas represent pulmonary expiratory air trapping secondary to obstruction of the small airways (Fig. 1).
High resolution computed tomography (HRCT) lung at T0 shows in the expiratory phase a marked and uneven alteration in density of both lungs for the coexistence of higher density and hyperlucency areas (part of the mosaic attenuation). These areas represent pulmonary expiratory air trapping secondary to obstruction of the small airways.
Bronchoalveolar lavage (BAL) fluid demonstrated an increase of total cells (2.3×106/ml), lymphocytes (55% of the total cells), and eosinophils (1%). CD4/CD8 ratio of lymphocyte surface markers was 0.4.
The results of BAL (marked lymphocytosis, CD4+/CD8+ ratio lower than one) confirmed the clinical suspicion of hypersensitivity pneumonitis.
So, after obtaining written informed consent, a 10-ml blood sample was collected from the antecubital vein, to perform IL-22 serum levels assay at T0 (Table 1).
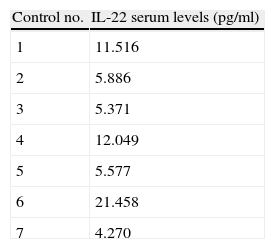
IL-22 serum levels in seven healthy subjects (Controls) and in patient suffering from hypersensitivity pneumonitis (HP) at T0 and at T1 (three months after removal from the workplace).
| Control no. | IL-22 serum levels (pg/ml) |
| 1 | 11.516 |
| 2 | 5.886 |
| 3 | 5.371 |
| 4 | 12.049 |
| 5 | 5.577 |
| 6 | 21.458 |
| 7 | 4.270 |
| Patient | IL-22 serum levels (pg/ml) |
| T0 | 2.460 |
| T1 | 0.868 |
The IL-22 serum levels were lower than the minimum of seven controls (2.460pg/ml vs. 4.270pg/ml).
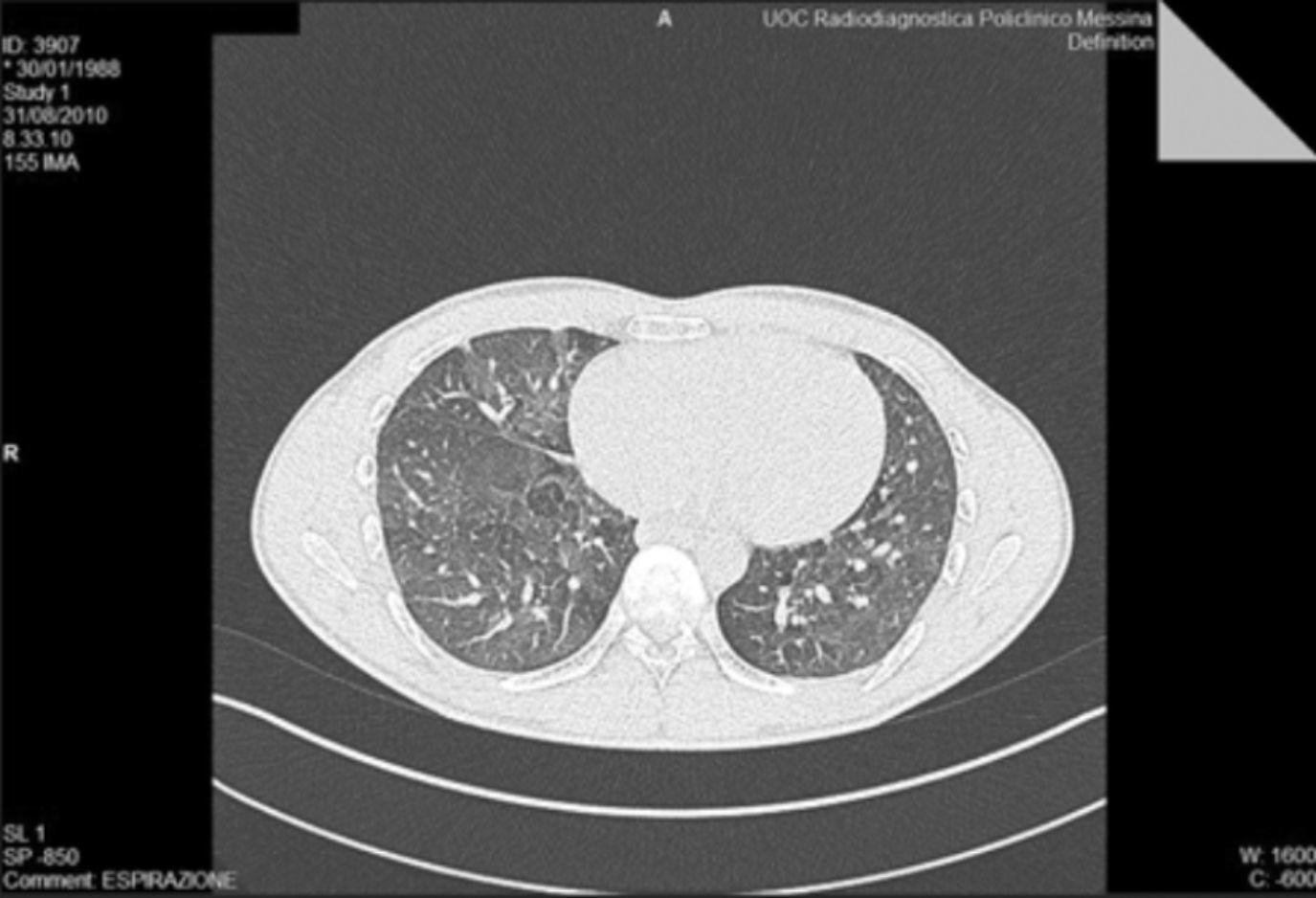
We removed the patient from his workplace and after three months we repeated the blood tests: the white blood cells, haematocrit, CRP, fibrinogen and serum iron were normalised, the HRCT scanning showed some images of the mantle micronodular lung parenchyma, no increase in size with respect to previous examination. No frosted glass aspect was observed, or inter- and intralobular septal thickening, or clumps of inflammatory nature. Nor was there detectable honeycombing, bronchiectasis or bronchiolectasis traction. The density of lung parenchyma during breathing was regular. This reveals only moderate diffuse abnormalities of the bronchial walls, which appear minimally thickened. This finding has not progressed from the previous examination (Fig. 2).
High resolution computed tomography (HRCT) lung at T1 shows some images of the mantle micronodular lung parenchyma, which do not increase in size than the previous examination. At the current appreciation we do not identify aspects frosted glass, or inter- and intralobular septal thickening, or clumps of inflammatory nature. We do not appreciate honeycombing, bronchiectasis or no bronchiolectasis traction. The density of lung parenchyma in breath is regular. It indicates only moderate diffuse abnormalities of the bronchial walls, which appear minimally thickened. This finding has not progressed from the previous examination.
Furthermore, the patient reported the disappearance of symptoms after only seven days off work.
We repeated the IL-22 assay at T1, the level was still lower than the minimum control (0.868pg/ml vs. 4.270pg/ml).
IL-22 has emerged as an important cytokine in mucosal immunity. The mechanisms by which IL-22 protects epithelial structures in the lung from damage induced by numerous environmental insults that are under intense investigation. Emerging data suggest that IL-22 may provide mucosal protection by inducing antimicrobial peptides from epithelial cells in the lung as well as maintaining epithelial integrity either by preventing injury or accelerating epithelial repair after a variety of insults.8
Although the IL-22 is reported to play a role in HP, the studies are limited to murine models; in our opinion this is the first report of involvement of this IL in a human patient suffering from HP.
We can hypothesise that the low IL-22 serum levels found in our patient at T0 were related to the patient's inability to respond to injury and could explain the patient's susceptibility to developing HP. The decrease in IL-22 serum levels, after three-month suspension from work in the greenhouse was, in our opinion, related to removal from the antigenic stimulus.
IL-22 could thus become a useful marker for understanding in advance who is at risk of HP, but other studies and larger series are needed.
We would like to thank Ms. A. Donato for the editing of the text.