INTRODUCTION
Food hypersensitivity (FH) affects between 1-2 % of adults. It is currently believed that actual prevalence is rising substantially similar to other atopic conditions such as asthma and allergic rhinitis. Many food allergies appear during childhood but some never develop tolerance to these food allergens throughout their life. This persistence can be associated with early onset, severity and accompanying atopy.
Adverse reactions to food items may result from enzyme deficiencies, exaggerated pharmacological or immunological responses that can be defined as Ig E mediated and non-Ig E mediated. The spectrum of food hypersensitivity ranges from cutaneous to potentially life threatening symptoms after ingestion1,2.
Seasonal allergic rhinitis (SR), a chronic disorder of nose and sometimes airways, is one of the most commonly seen allergic diseases, which is totally Ig E-mediated. It is a disease of younger age groups which tends to resolve spontaneously in the middle age. The capacity of pollens sensitizing the patients is theoretically universal, but the nature and number of pollens varies with geography, temperature and climate. The main cause of seasonal rhinitis seems to be pollen sensitivity in Turkey according to recent studies3-11.
Patients with allergic rhinitis/conjunctivitis due to birch and, to a lesser extent, other Betulaceae (hazel, alder) pollen are frequently allergic to tree nuts, fruits and vegetables, including apples, carrots and potatoes. Most patients develop mild symptoms but anaphylaxis may occur due to these cross-reacting food. Some birch or hazel pollen allergens cross-react with those of apple, other fruits or various nuts (almonds, apples, apricots, buckwheat, carrots, celery, cherries, coriander, fennel, hazel nuts, honey, kiwi, nectarines, parsley, parsnips, pears, peaches, peanuts, peppers, plums, potatoes, prunes, spinach, walnuts and wheat)12,13.
In the classification of FH, the oral allergy syndrome has a fascinating place. It has more recently been renamed pollen-food allergy syndrome. Hay fever patients sensitized to pollen develop oral allergic symptoms to certain fruits and vegetables, which occur in up to 40 % of all hay fever sufferers who are allergic to birch or grass pollen. Once sensitized to the pollen, they develop allergy to similar allergens found in fresh fruit and vegetables. Symptoms occur within a few minutes of contact and are almost always localized to the mouth and oro-pharynx with lip and oral itching. Oral swelling with occasional laryngeal edema may ensue. However, patients do not react to cooked fruit or vegetables14.
Our aim was to determine the frequency of the FH and the risk factors for developing hypersensitivity to foodstuff in patients with SR in an adult allergy clinic in a 13 year period, retrospectively.
METHODS
A retrospective study was conducted based on clinical records of 955 patients diagnosed with seasonal rhinitis at our clinic between 1 January 1991 and 31 December 2003. Out of 955 patients, 774 were enrolled since complete data were available only for these. The collected data included demographic features, allergen spectra, initial symptoms, allergic accompanying diseases and accountable food allergens. Risk factor analysis was performed for patients with FH.
Atopy and allergen spectra was assessed by skin prick test (SPT) reactions to 17 common aeroallergens, prepared by firms ALK (Denmark), Stallergens (France) and Greer (USA) (Dermatophagoides pteronyssinus, Phleum pratense, Olea europa, Artemisia vulgaris, Parietaria officinalis, Corylus avellena, Betula verrucosa, Populus alba, cat, dog, horse, Alternaria alternata, Cladosporium herbarum, Aspergillus fumigatus, cockroach, Apis mellifera, Vespula species). Food panel (including bean, tomatoes, hazelnut, onion, parsley, corn, pea, carrot, egg, nut, shrimp, cherry, sheep, mackerel, orange, peach, soybean, mussel extracts) has been performed in patients whom were thought to have anamnestic food allergy and/or intolerance. Tests were carried out as described by Österballe et al15 by pricking the skin on the volar face of the forearm with a special lancet. Histamine and saline were used as positive and negative controls, respectively. Resulting wheals were measured after 15 minutes. A positive reaction was defined as a wheal with a geometric mean diameter of at least 3 millimeters. Prick test positivity was defined as a positive response to at least one of the aeroallergens used. Prick tests were not performed in cases of pregnancy, dermographismus and antihistaminic usage.
SPSS MS Windows Release 10.0 (SPSS Inc.) was used for statistical analysis. Personal characteristics and disease related factors were compared between food hypersensitivity and allergic rhinitis patients. Chi-square testing with continuity correction and student's t-test were used for categorical and continuous variables, respectively. Association of the factors with FH was adjusted for age and gender. Age, duration of rhinitis and duration of rhinitis symptoms were categorized into two groups according to the medians of these parameters. Odds ratios were used to assess the strength of the association between FH and characteristics of the patients. Final logistic regression model was developed to assess the independent association between FH and the factors which were significantly associated with FH in the adjusted analysis. Statistical significance was defined for p values less than 0.05.
RESULTS
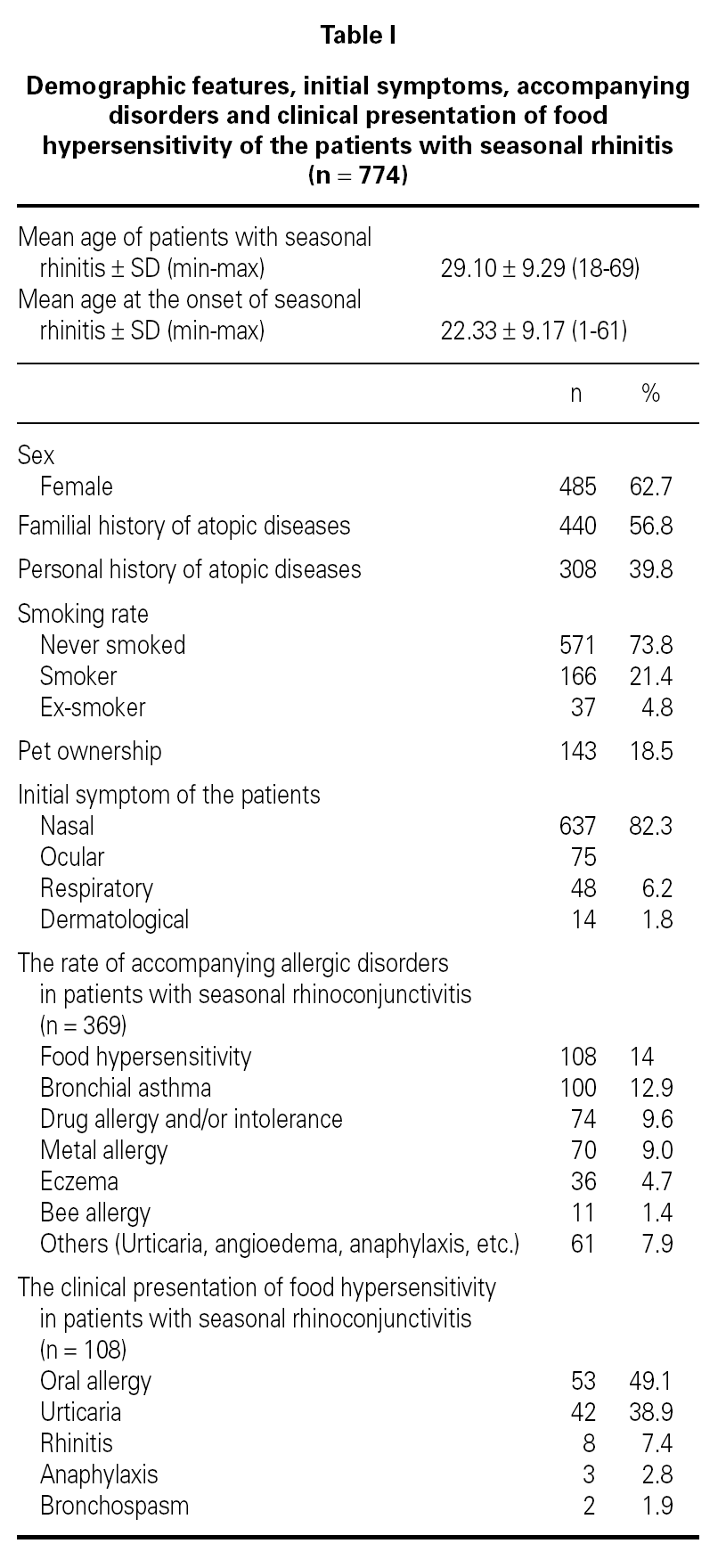
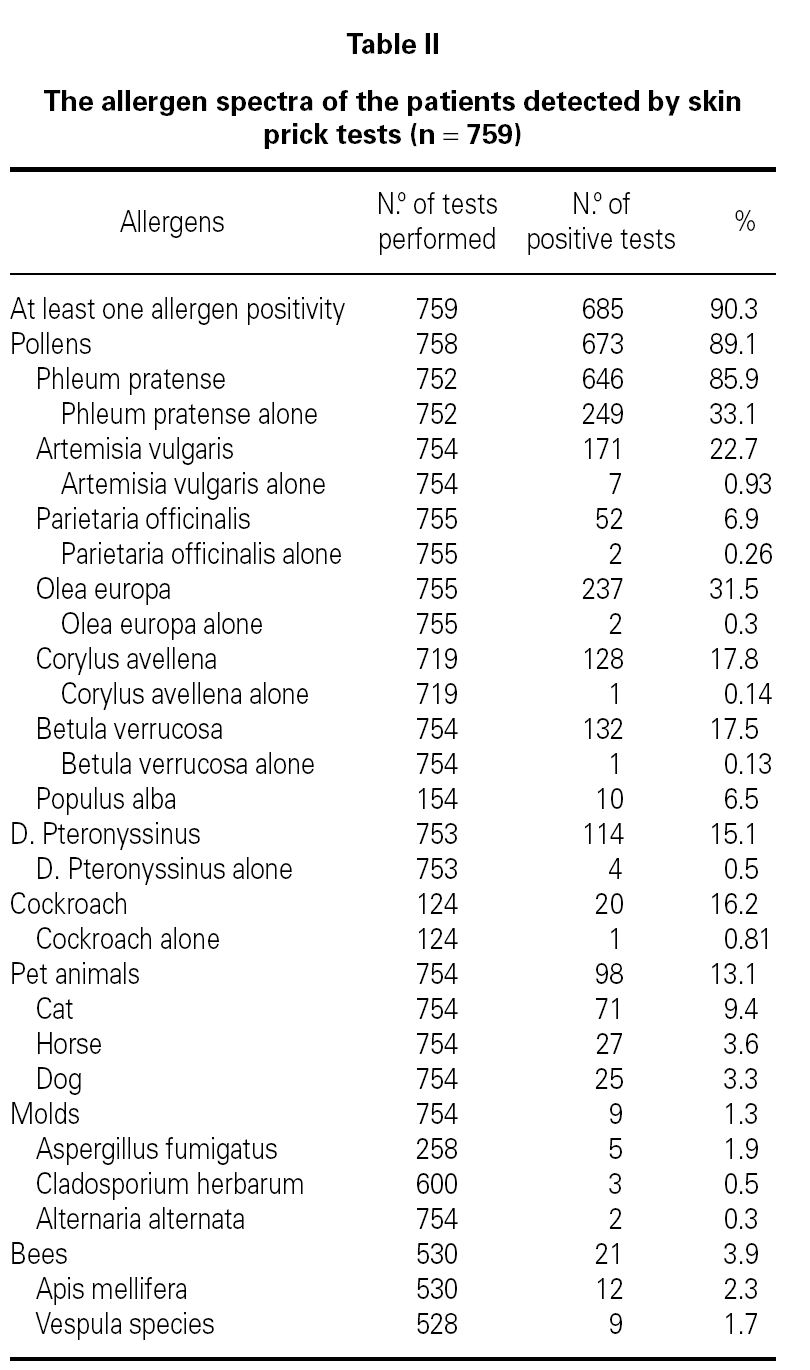
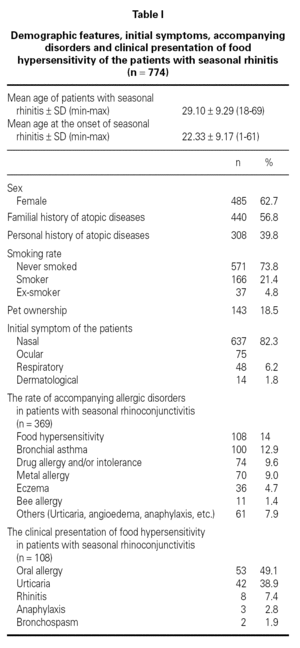
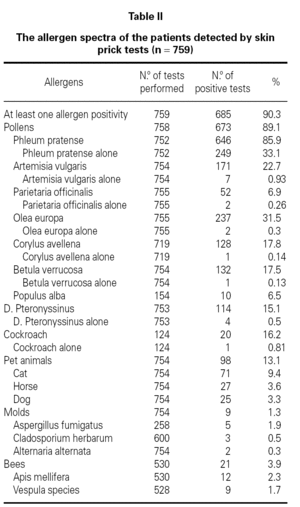
Demographic features, initial symptoms and accompanying disorders of the patients are given in table I. The mean age of the patients was 29.1 ± 9.29 and 485 of them were females. Distribution of patients regarding age, sex, smoking status are shown in table I. The major initial complaints of the patients were due to nasal symptoms in 82.3 %, respiratory symptoms in 9.7 %, cutaneous symptoms in 6.2 %, and ocular symptoms in 1.8 % (table I). The mean age at the onset of SR was 22.3 ± 9.2 and the mean duration of SR was 6.8 ± 6.8 years (median 5 years). The patients have been symptomatic with a mean of 3.5 ± 1.7 months (median 3 months) in a year. SPT were positive in 685 out of 759 (90.3 %) patients who were tested and 267 patients were sensitized to single allergen. The allergen spectrum is shown in table II.
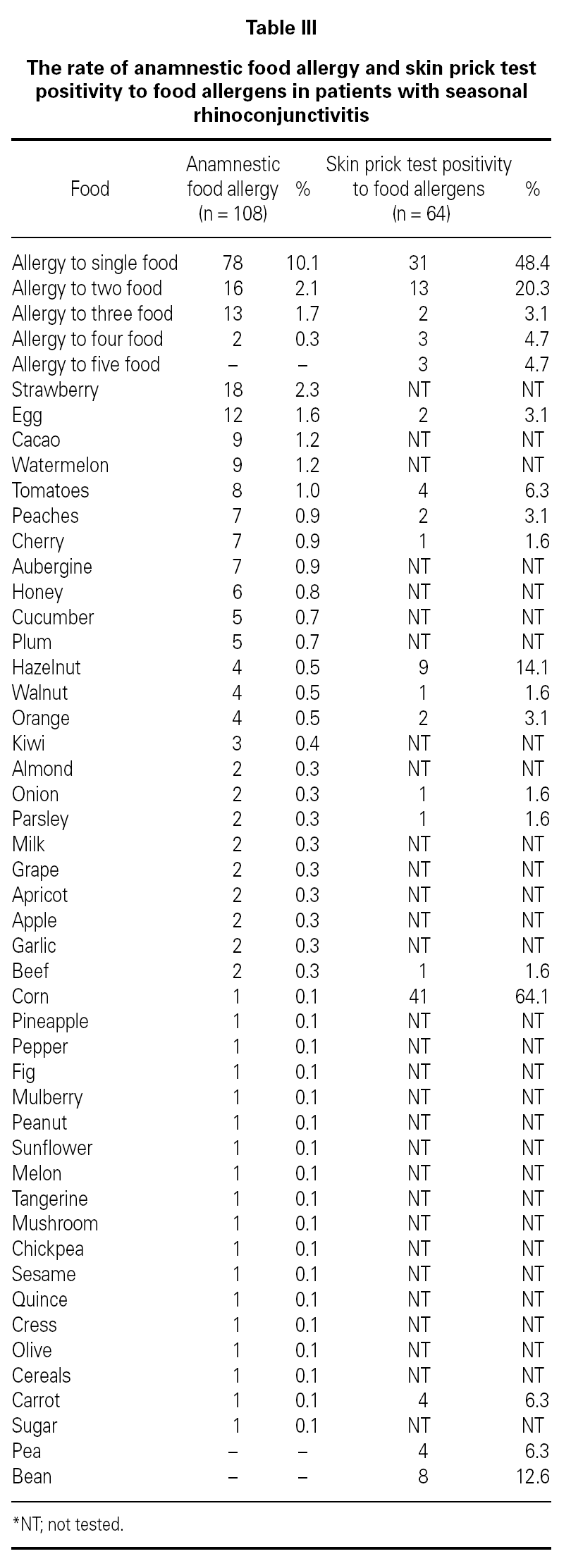
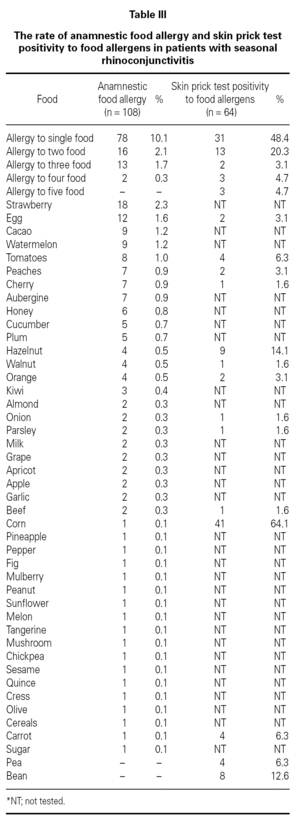
Three hundred sixty-nine (47.7 %) patients had at least one allergic accompanying diseases which were FH (14 %), asthma (12.9 %), and drug allergy and/or intolerance (9.6 %). One hundred-eight patients (14 %) had anamnestic food hypersensitivity due to at least one kind of food (strawberry, egg, cacao, watermelon, tomatoes, peaches, cherry, aubergine, honey, cucumber, plum, hazelnut, walnut, orange, kiwi, almond, onion, parsley, milk, grape, apricot, apple, garlic, beef, corn, pineapple, pepper, fig, mulberry, peanut, sunflower, melon, tangerine, mushroom, chickpea, sesame, quince, cress, olive, cereals, carrot, sugar, pea, bean). We performed SPT with food allergens in 64 of 108 patients who were available for the test (table III). There were only 3 (4.7 %) patients in whom the prick test positivity was compatible with anamnesis. Although the patients had the most common allergic symptoms due to strawberry by history, the most frequent sensitivity determined by SPT was against corn.
The clinical presentations of FH were oral allergy (49.1 %), urticaria (38.9 %), rhinitis (7.4 %), anaphylaxis (2.8 %) and bronchospasm (1.9 %), respectively (table I). There were only three patients with life-threatening anaphylactic reactions. Anaphylaxis developed due to honey in two patients and due to mushroom in one patient. Two patients presented FH with bronchospasm, which occurred due to watermelon in one patient, and due to orange in another.
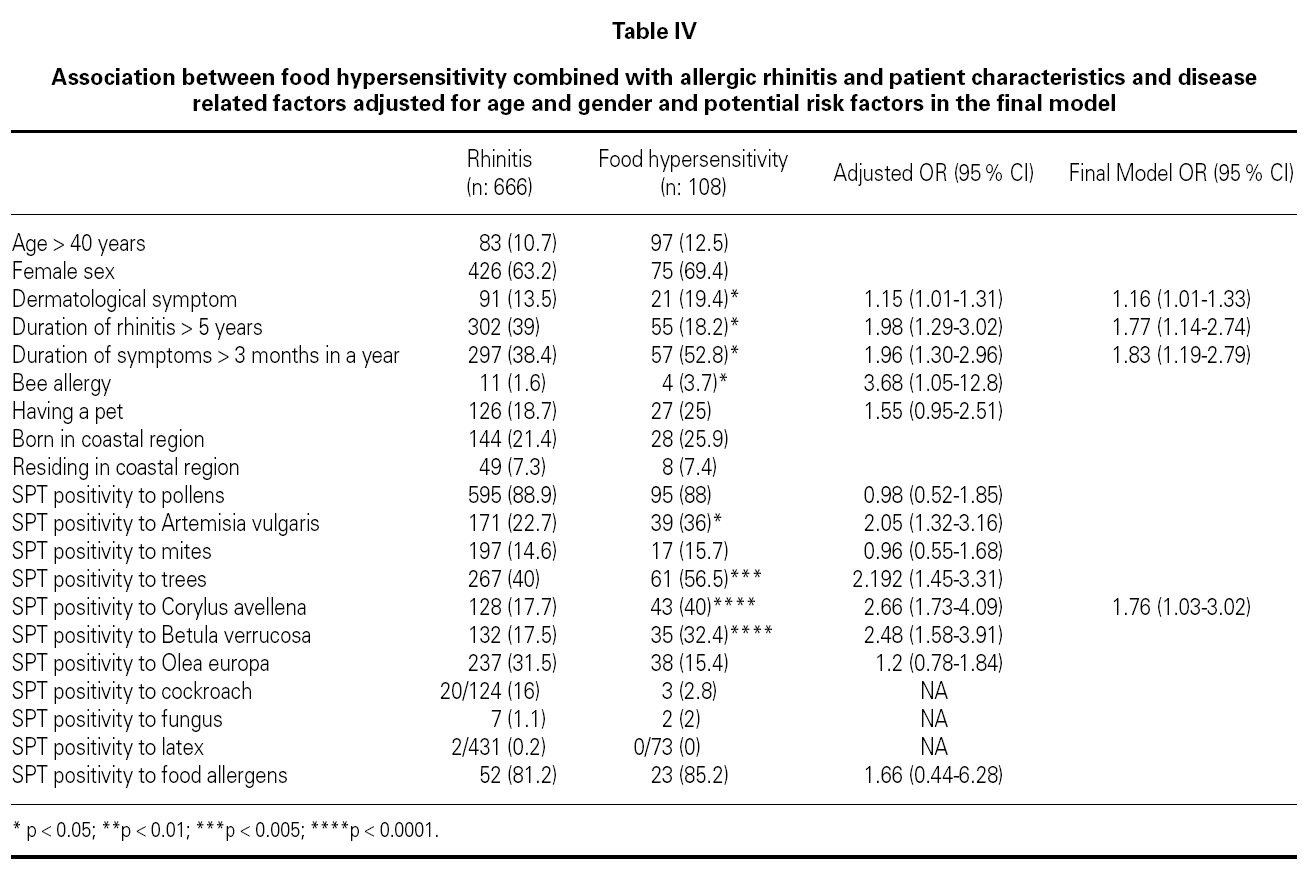
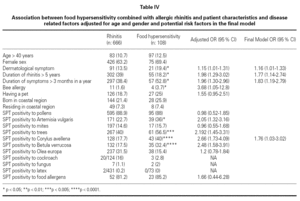
Association between FH combined with allergic rhinitis and patient characteristics and disease related factors adjusted for age and gender and potential risk factors were examined in the final model (table IV). The final model included dermatological symptoms (OR; 95 %CI) (1.16; 1.01-1.33), duration of rhinitis > 5 years (1.77; 1.14-2.74), duration of symptoms > 3 months in a year (1.83; 1.19-2.79), SPT positivity to Corylus (1.76; 1.03-3.02), which were associated with the development of food hypersensitivity in patients with SR. Bee allergy (1.4 % of patients with seasonal rhinitis) (3.68; 1.05-12.8), skin test reactivity to Artemisia vulgaris (2.05; 1.32-3.16), tree pollens (2.19; 1.45-3.31) and Betula verrucosa (2.48; 1.58-3.91) were associated with FH after the adjustment for age and gender, but not included in the final model. Skin test reactivity to Artemisia vulgaris (2.71; 1.19-6.13) and Corylus avellena (3.12; 1.20-8.12) were associated with strawberry allergy and skin test reactivity to Artemisia vulgaris (3.59; 1.14-11.36) was associated with tomato allergy, but skin test reactivity to Phleum pratense (0.31; 0.098-0.98) was less frequent in patients with watermelon allergy after the adjustment for age and gender.
DISCUSSION
Even though not a life threatening disease, seasonal allergic rhinitis has a remarkable social impact and can not be left to its natural course. Pollens appear seasonally and reflected in the symptoms of seasonal allergic rhinitis in patients living in temperate climates. Recent studies show that grass pollen in Turkey3-11, birch and grass pollens in northern Europe16-20, ragweed pollen in America21; and mugwort and olive pollens in Mediterranean area22-26 are the most common and important allergens for these regions. This survey shows that the atopy rate and allergen spectra detected by SPT was 90.3 % and the most common sensitizing allergen was grass pollen in patients with SR in Ankara.
Although the patients with SR have mainly rhinitis symptoms (major complaints being nasal in 82.3 %), they might have some other accompanying allergic conditions like food hypersensitivity, asthma, and drug allergy and/or intolerance. The FH (14 %) was the most common accompanying allergic disease among 369 (47.7 %) patients who had at least one accompanying allergic disease (food hypersensitivity, and/or astma, and/or drug allergy and/or intolerance). This is a retrospective clinical study. If we could conduct a prospective survey and perform more sophisticated tests (such as some provocation tests or detailed specific IgE analysis), the frequency of the patients with food hypersensitivity might have been increased.
There is a substantial gap between perceived and proven FH. Careful history taking and physical examination remains the cornerstone of the diagnosis of food hypersensitivity. Symptoms were predominantly limited to oropharyngeal mucosa and skin but progression was reported in 5 patients; 3 developed anaphylactic reactions and 2 had bronchospasm. An immediate, systemic reaction mediated by IgE is called anaphylaxis irrespective of severity, but colloquially the term anaphylaxis is applied to severe, potentially fatal allergic reactions. Previously-identified, food-allergic (particularly to peanut, tree nuts, and seafood) adolescents with underlying asthma seem to have the highest risk of death27. In this study, two patients exhibited anaphylactic symptoms after the ingestion of honey and one patient after the ingestion of mushroom. While apparently uncommon, allergies to honey have been reported and can involve reactions varying from cough to anaphylaxis which are not so rare28. Recently, the incidence of honey allergy was reported to be 2.3 % of a group of 173 patients with food allergy. In the literature, allergy to honey is often attributed to the highly allergenic pollen content that the bees have collected and other allergens, most likely of bee origin29-32. Studies support the hypothesis of a strict link between sensitization to Compositae and adverse reactions to honey33,34, as one of our patients was sensitized to Artemisia vulgaris and the other to Parietaria officinalis. For the other anaphylactic reaction due to ingestion of mushroom, currently there is no available data in the patients with seasonal rhinitis.
Bronchospasm is another important life threatening feature of FH. There were two patients in our study presented FH with bronchospasm, which occurred due to watermelon in one patient, and due to orange in another. Few studies have evaluated IgE-mediated hypersensitivity to melon showed that isolated melon allergy is rare, with most patients either having allergic rhinitis, asthma, or both and associated food allergy35. Although there were studies about asthmatic reactions against orange juice, there is currently no available data about orange fruit induced bronchospasm in patients with SR.
FH by history and the results of SPT performed with food allergens were discordant. FH might not have been totally Ig-E mediated in contrast to SR. It is well-known that the golden standard in the diagnosis of food hypersensitivity is double-blind oral provocation testing36. Except for the academic studies, it is obvious that there is no need to perform prick tests or specific IgE with food allergens in routine daily practice. This result stresses the general knowledge that prick tests with food have limited diagnostic value for food allergy and/or intolerance.
FH and SR are infrequently associated conditions. There are limited data for FH risk factors among children and adults. The factors that progress seasonal rhinitis into hypersensitivity to food are not clear. Analysis of our data suggested dermatological symptoms, duration of rhinitis > 5 years, duration of symptoms > 3 months in a year; and skin prick test reactivity to Artemisia vulgaris, Betula verrucosa and Corylus avellena as risk factors for the development of FH in patients with SR. Patients with allergic rhinitis/conjunctivitis due to tree and weed pollens are frequently allergic to several foodstuff, which points out the importance of Betulaceae (hazel, alder) pollens for the development of FH in patients with SR in Turkey. History and allergen spectrum of patients should carefully be evaluated in order to determine the risk factors for developing FH in patients with SR. As this was a cross-sectional study it is not possible to assess the temporal association and causality of the associations. A prospective study of patients with SR would help to elucidate our findings.
In summary, seasonal allergic rhinitis lasts approximately 3.5 months and the main cause is grass pollen sensitivity in Ankara, Turkey. FH (14 %) was the most common accompanying allergic disease in patients with SR. Prick tests with food have limited diagnostic value for food allergy and/or intolerance. History and allergen spectrum of patients should carefully be evaluated in order to determine the risk factors for developing FH in patients with SR.