INTRODUCTION
Allergenic extracts have been used in the treatment of allergic rhinitis for over 90 years1,2, its efficacy being demonstrated in several randomised and controlled studies3. The goal of this therapy is to alter immunologic reactivity to allergens, trying to either induce tolerance or a T1-type reactivity to allergens which could antagonize the characteristic T2-type allergic reactivity. Moreover, SIT is the only treatment that can alter allergic rhinitis' natural history4 and prevent the emergence of new sensitisations5,6. Glutaraldehyde-modified extracts have been introduced in order to reduce IgE-binding capacity and therefore systemic adverse reactions, while using higher allergen doses and therefore enhancing immunoregulatory activity of this therapy7-9. It was the objective of our study to determine the efficacy of SIT with a depigmented, glutaraldehyde modified Dermatophagoides pteronyssinus extract in the treatment of persistent allergic rhinitis patients, assessing subjective and objective parameters during a period of treatment of 12 months and trying to determine if there were early clinically significant improvements, comparing pre and post-treatment values as well as comparing the results of SIT-treated and control patients. As this extract contains a 10 times more concentrated mite extract, we additionally evaluated the incidence of clinical adverse local or systemic reactions after SIT injections.
MATERIAL AND METHODS
Study Design
This study was designed and conducted as a pragmatic, open clinical trial, in order to evaluate under real-world conditions the safety and effectiveness of the treatment using objective and subjective criteria10,11, as well as to evaluate how much time was needed for the onset of the expected clinical effects.
The study was controlled, parallel and included a random allocation of the patients to each one of the groups.
Patients
We included 50 moderate-severe persistent allergic rhinitis patients, monosensitized to mites (Dermatophagoides), as suggested by clinical history, skin prick tests and serum specific IgE. There were 27 females and 23 males, mean age 23.6 ± 8,14 years (range: 15-46 years), which were randomly assigned either to a control group which did not receive specific immunotherapy or to a SIT group which received injections of a modified Dermatophagoides pteronyssinus extract with a monthly dose of 55 μg (annual dose = 710 μg). All patients were allowed to take oral antihistamines and/or nasal steroids when needed. This study was approved by the Ethical Committee of Lisbon Faculty of Medicine and patients gave their written informed consent.
Patients were assessed three times during the year of the study: one initial visit (T0) before the start of SIT, an intermediate visit at 6 months (T1) and a final visit at 12 months (T2).
Visual Analog Scale (VAS)
At the beginning of each visit patients were asked to mark on a VAS with 10 cm long the point which would best correspond to the intensity of their rhinitis symptoms during the past month.
Symptom Score
During the week that preceded each visit patients were asked to fill a diary card regarding daily intensity of nasal symptoms (sneezes, itching, rhinorrea and nasal obstruction), eye symptoms (pruritus and tears) and bronchial symptoms (cough, wheezing and dyspnea). Each symptom was graded from 0 (no symptoms), 1 (mild), 2 (moderate) or 3 (severe, incapacitating symptoms) and the symptom score was obtained by the sum of all symptoms.
Specific Nasal Challenge
It was performed in each visit with a lyophilised Dermatophagoides pteronyssinus allergenic solution (CBF LETI Laboratories, Spain), which was reconstituted 30 minutes before provocation in order to obtain a solution with an allergenic potency of 100 HEP/ml (Histamine Equivalent Potency). This solution was serially tenfold diluted in order to obtain four other allergenic solutions with 10 HEP/ml, 1 HEP, 0.1 HEP and 0.01 HEP, respectively. We did not use any vasoconstrictor medication before nasal challenge and patients were asked not to use any nasal steroids during the two weeks and antihistamines during the three days that preceded nasal challenge.
Nasal provocation was always performed in the same room and by the same investigator. It started with nebulization of 0.05 ml of negative control, followed by 0.05 ml nebulizations of the allergenic solutions (20 minutes interval) with increasing potency. Nasal challenge was stopped when the sum of a nasal score (sneezes, itching, rhinorrea, nasal obstruction; 0-no symptoms, 1-mild, 2-moderate, 3-severe) was superior or equal to 6 (maximum 12). Allergenic threshold was therefore the potency of the last allergenic solution administered.
Specific immunotherapy (SIT)
We used a Dermatophagoides pteronyssinus extract, depigmented and polymerised with glutaraldehyde (DEPIGOID®, CBF LETI Laboratories, Spain), described in detail elsewhere7.
All SIT-treated patients received a build-up phase with 5 weekly injections of increasing doses (1, 5, 11, 33 and 55 μg of the extract) and a maintenance phase with 11 monthly doses of 55 μg each. SIT was administered according to European Academy of Allergology and Clinical Immunology guidelines12.
Safety assessment
All SIT-treated patients remained in the Hospital for 2 hours after each injection and we recorded any reaction that occurred during this period. If there was any late reaction, patients were instructed to make a phone contact with the Hospital and, if necessary, to take the adequate medication.
Statistical analysis
Comparisons between groups were performed by the Mann-Whitney rank sum test and comparisons within the same group were performed by the Wilcoxon-signed-rank test.
RESULTS
All SIT-treated patients completed the treatment schedule. We observed local reactions of pruritus and edema (induration diameter > 10 cm) in 28 % of the patients, in a total of 24 reactions (6 % of a total 400 injections). These reactions were mild and did not need any systemic treatment. In the build-up phase one patient presented an immediate systemic reaction consisting of generalized pruritus and erythema of the face and neck, without papules. There were no respiratory or cardiovascular changes and this reaction was successfully treated with antihistamines. No further reactions were observed in this patient.
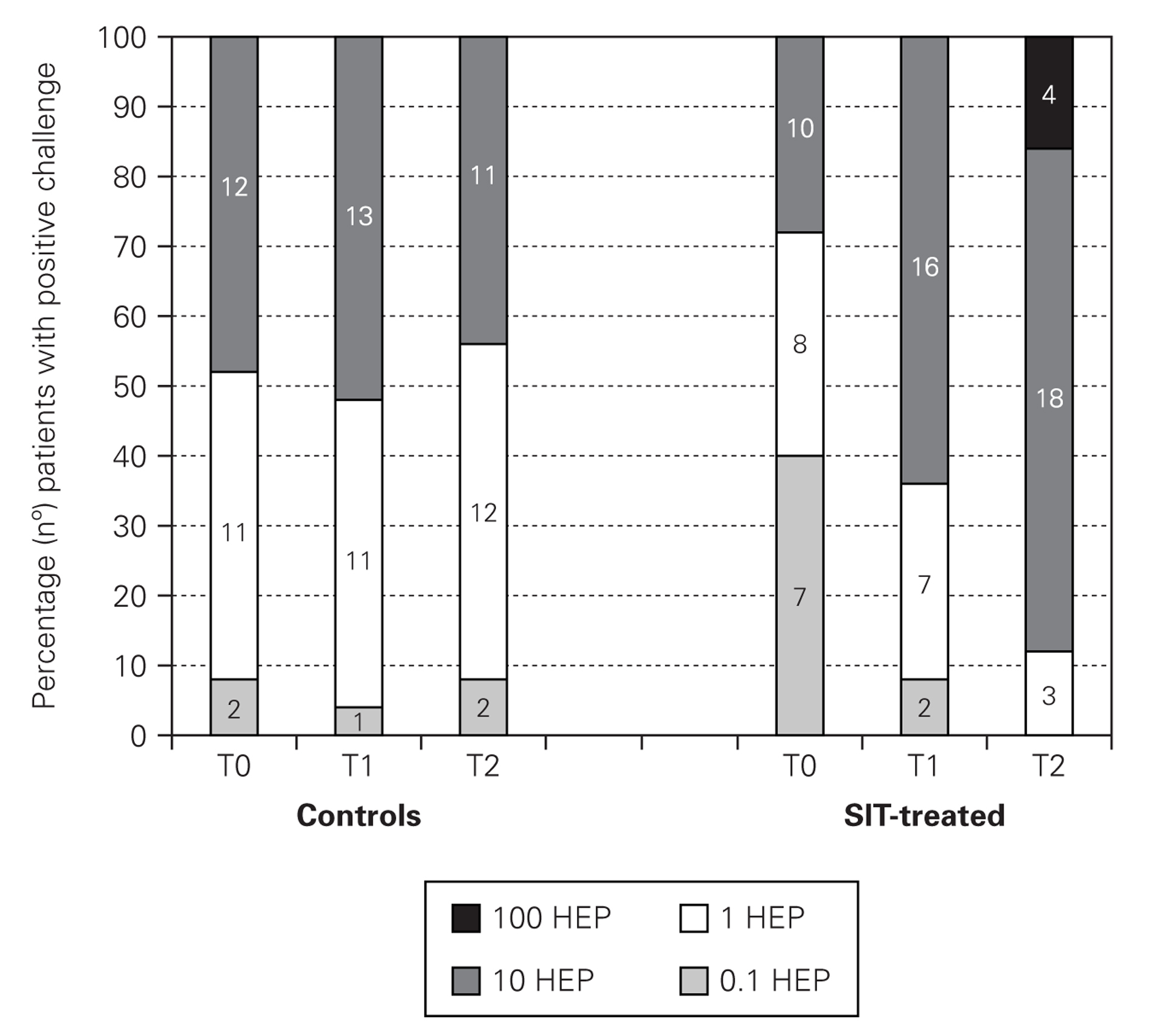
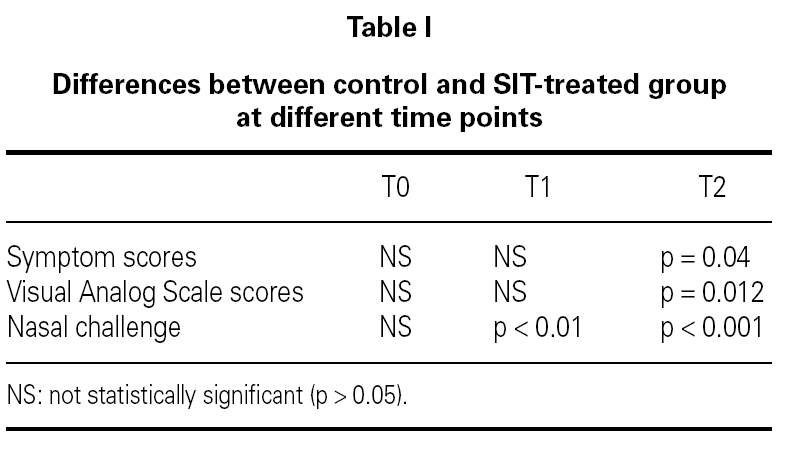
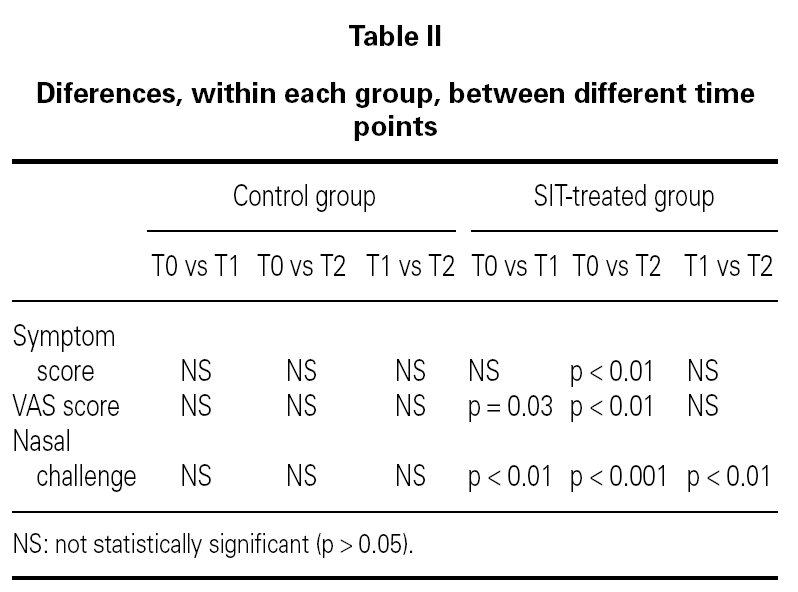
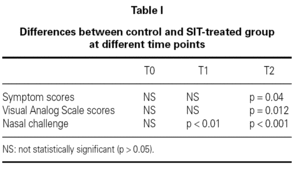
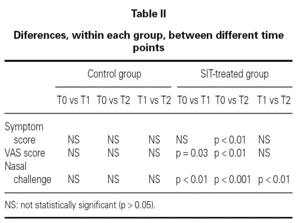
In nasal challenges we have observed a progressive increase in allergenic concentration tolerated by SIT-treated patients, while in the control group there were no significant differences. In the control group, the mean concentration that induced a positive nasal challenge was 5.24 ± 4.66 HEP, in the beginning of the study, 5.64 ± 4.63 HEP at 6 months and 4.88 ± 4.63 HEP in the end, without any change in the distribution of the allergenic thresholds (fig. 1). On the contrary, in the SIT-treated group there was a progressive increase in the mean allergenic concentrations needed to obtain a positive nasal challenge from 4.34 ± 4.72 HEP at the beginning of the study to 6.68 ± 4.51 HEP after 6 months and 23.32 ± 34.28 HEP at the end of the study. In the distribution of the allergenic thresholds (fig. 1) we could clearly see this improvement in allergenic tolerance in SIT-treated patients, with disappearance of positive challenges with lower allergenic concentrations (0.1 HEP), appearance of some patients who had positive challenges only with the highest concentrations (100 HEP) and an 80 % increase in the number of patients who had a positive challenge only with a relatively high concentration of the allergenic solution (10 HEP). These differences were statistically significant immediately after six months' treatment, whether comparing the evolution within the SIT-treated group (before and after treatment) or comparing SIT-treated and control groups (tables I and II).
Figure 1.--Allergenic thresholds in nasal challenges.
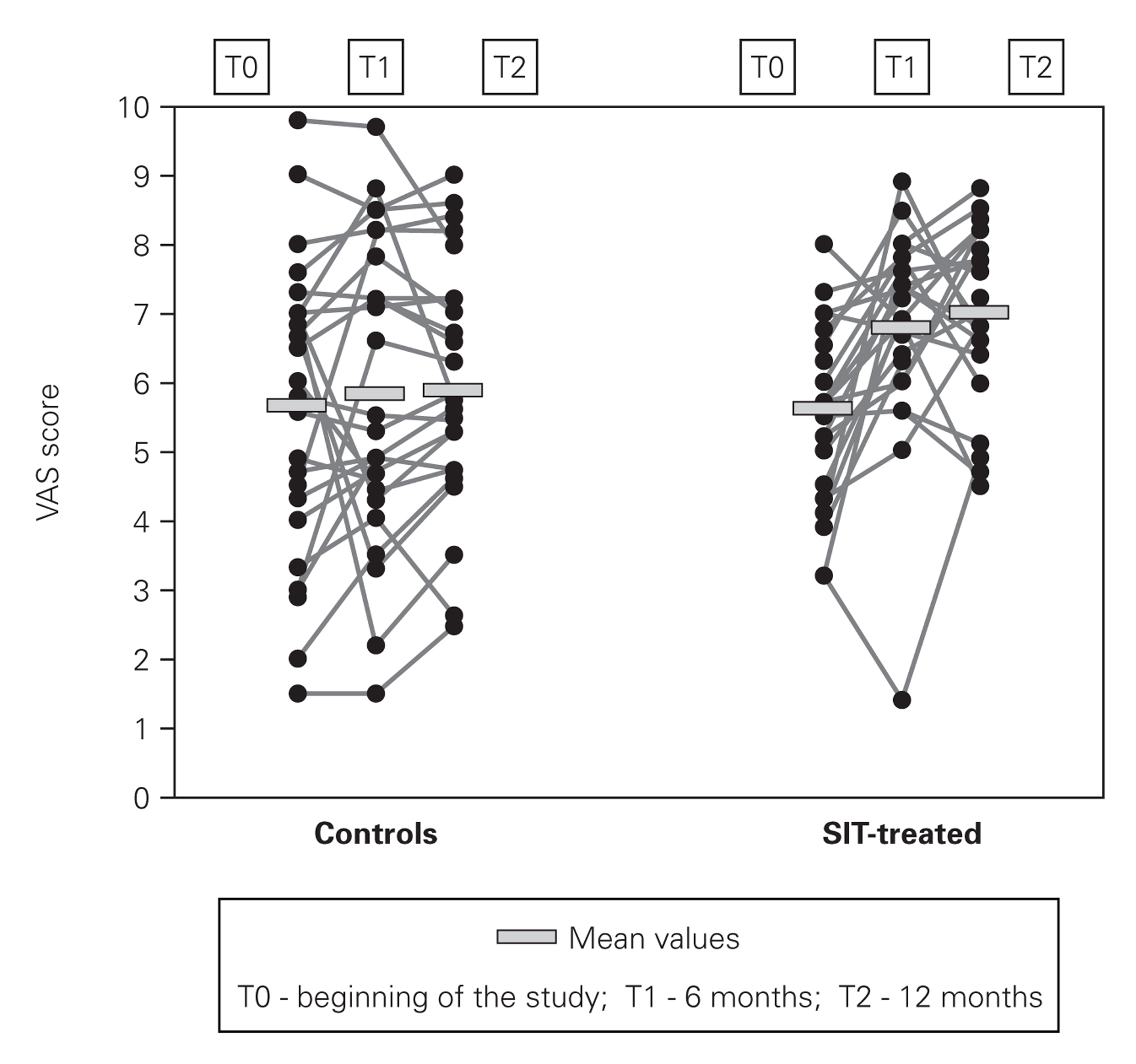
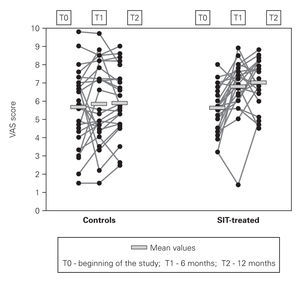
In VAS (fig. 2) we have also observed progressive improvement in the mean scores of SIT-treated patients from 5.61 ± 1.33 cm at the beginning of the study to 6.80 ± 1.46 cm at 6 months and to 6.99 ± 1,21 cm at the end (24.6 % increase), while in the control group there was only a very slight and non-significant increase in these scores (from 5.67 ± 2.12 cm to 5.88 ± 1.74 cm at the end of the study).
Figure 2.--Visual analog scale (VAS) scores.
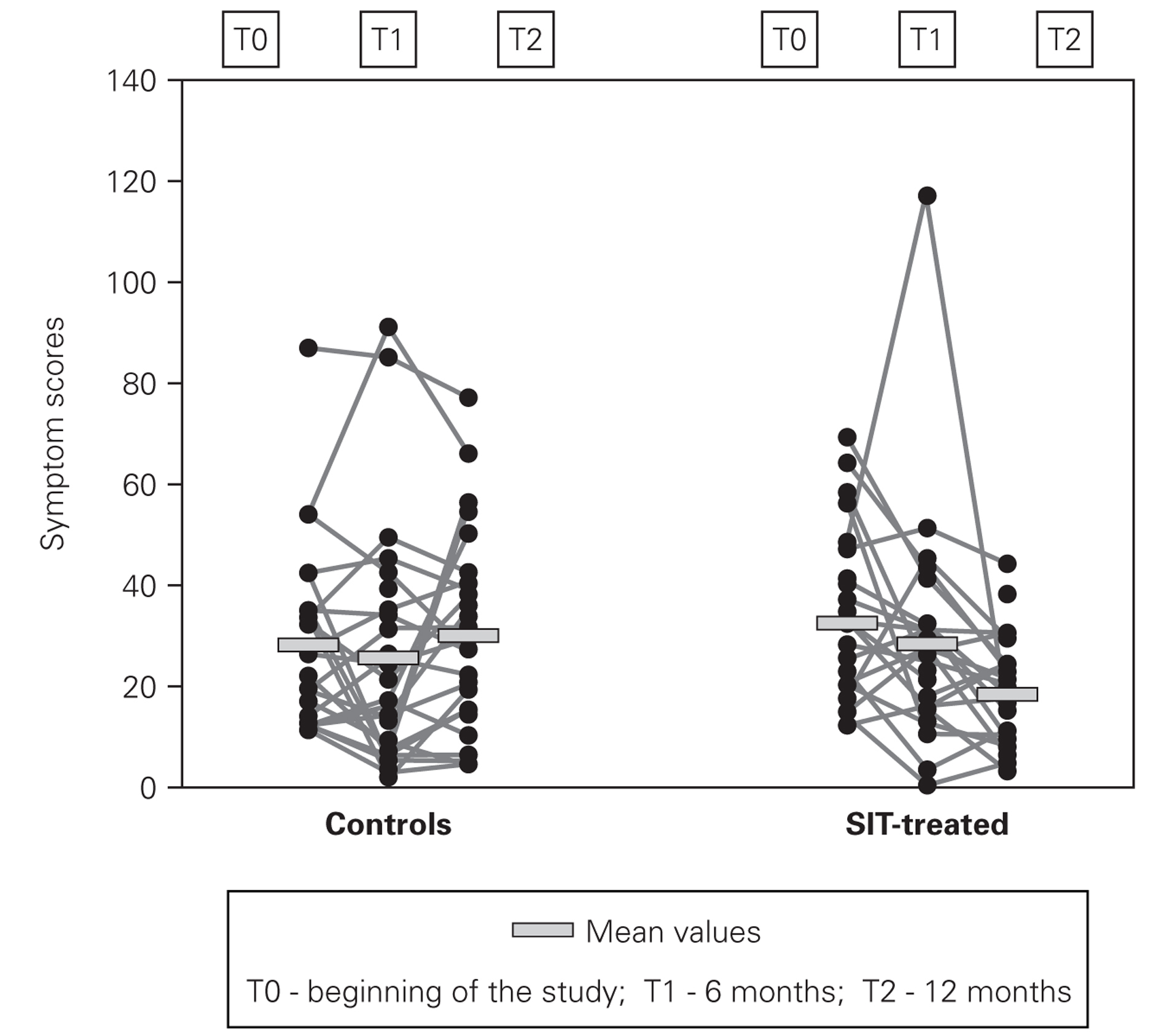
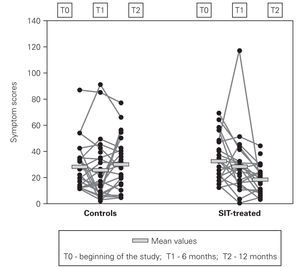
In symptom scores there was also progressive improvement, however being more pronounced in the second semester of the study (fig. 3). Mean symptom scores in SIT-treated group decreased from 32.2 ± 16.9 to 28.3 ± 22.3 at 6 months and 18.3 ± 10.5 at the end of the study, which represents a mean reduction of 43.2 %. In the control group despite a small decrease at 6 months, at the end of the study these scores were slightly higher than in the beginning (27.8 ± 18.0 and 29.8 ± 19.9).
Figure 3.--Individual one-week symptom scores.
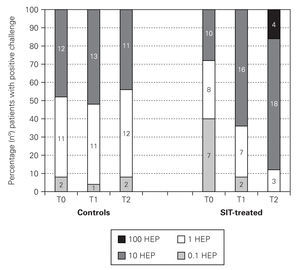
In both VAS and symptom scores there were statistically significant differences after 12 months treatment whether comparing the evolution within the SIT-treated group (before and after treatment) or comparing SIT-treated and control groups (tables I and II).
DISCUSSION
In this one-year open, pragmatic study we describe the clinical effectiveness and safety of a depigmented polymerised vaccine of 100 % Dermatophagoides pteronyssinus in patients with moderate-to-severe persistent allergic rhinitis. Although having their own limitations due to their unblinded nature, pragmatic clinical trials are used to examine the benefits of a treatment under the real-world conditions that patients experience when following a specific treatment. As placebo-controlled trials, randomisation at the outset is the rule to include comparable subjects across treatment groups, but blinding is not recommended in pragmatic trials because it is not characteristic of the real world and may significantly alter some of the advantages and effectiveness of the treatment due to the confidence between patient and physician. Furthermore, pragmatic clinical trials are gaining wider acceptance in the evaluation of cost-effectiveness parameters in the treatment of allergic diseases13,14 and have already been used in the evaluation of this type of extract7. In this study we analysed the speed of effect using objective criteria such as nasal provocation tests and subjective criteria such as symptom and VAS scores.
One of the difficulties in accurately assessing SIT efficacy resides in the choice of which parameters should be evaluated and how their changes should be valorised. Some authors defend only two efficacy criteria: severity of symptoms and medication use, since these items are the only aspects that matter to patients. However, this approach that does not take into account either the advances in knowledge of allergic disease or the objective changes that have been shown to occur in double-blind, placebo controlled SIT studies. Although introducing more criteria may raise new questions when contradictory results are found in different evaluations, it is licit to use valid objective measures to question the accuracy of subjective evaluations15. Besides the intrinsic subjectivity of diary cards in real life practice, where there are no double-blind procedures, one other problem in evaluating intensity of symptoms or medication use in long periods of time through diary cards is the frequent inaccurate filling of these cards. For that reason, in this work we have chosen to obtain just one week's diary card. This is a debatable choice but has already been suggested by other authors in mite-allergic patients16. We chose a VAS score as a more global subjective approach and, as others17-19 have found a good correlation between these two methods (Spearman's rho = 0.547). As an objective approach we selected allergenic thresholds of a positive nasal provocation because they are well validated and have been used in several studies of SIT efficacy with mite extracts20,21.
In our study we have found that SIT with this modified mite-extract, that allows a schedule with injection of high allergenic doses already in the second month of therapy, improved significantly the VAS and precedent week's symptom scores at 12 months' treatment. Both these improvements were statistically significant when comparing pre and post-treatment measurements or when comparing SIT-treated and control patients. Other studies have also found early significant improvements in subjective parameters of SIT-treated patients16,19 and the mean decrease of 43 % in symptom scores of our patients is well above the 30 % efficacy limit suggested by Malling22, a fact which again underscores SIT efficacy at 12 months. But perhaps even more important was the finding that SIT could induce objective changes in target-organ specific reactivity and that, in this case, these changes were statistically significant immediately after a 6 months' treatment period, further increasing after 12 months, when a mean 5-fold increase in allergenic concentration was needed to obtain a clinically positive nasal provocation, in comparison with pre-SIT findings. Other authors, using comparable cumulative doses of this extract (523,5 mg) have also found a decrease in bronchial reactivity immediately after 6 months' treatment: SIT-treated allergic asthmatic patients had a significant increase in allergenic doses needed to obtain a 20 % reduction in FEV1 values, while control patients did not show any changes7. Apart from being an objective marker, the decrease in nasal reactivity is obviously of relevance to patients, since it means that some unavoidable daily-life contacts with mite particles will have much lesser consequences on symptoms. This has obvious direct implications in the subjective assessment of the patient's well-being.
Regarding safety, and although in this study we evaluated only a small number of patients/injections, we did not observe any severe systemic reactions and the incidence of local or systemic reactions was not higher than what is usually seen with conventional SIT extracts and schedules.
In view of the data here presented we believe that this study clearly demonstrates the early efficacy of SIT with polymerised and depigmented mite extracts in subjective and objective parameters relevant to allergic rhinitis patients.
CONCLUSIONS
In moderate-severe persistent mite-allergic rhinitis, SIT with a polymerised and depigmented mite extract, using a schedule that allows reaching high-dose maintenance therapy in approximately one month, appears to be a relatively safe treatment, which can rapidly (6 months) induce significant improvement in nasal allergenic reactivity. This is accompanied by reduction of symptom scores and improvement in subjective self-evaluation of the disease through a visual analog scale (with significant differences at 12 months' treatment), both parameters reflecting also a general improvement in these patients' well-being.