Rapid weight gain has been recently associated with asthma at school age, but its influence in respiratory symptoms during infancy is still unknown.
MethodsAnswers from 6541 parents living in six different cities of Brazil to the International Study of Wheezing in Infants (EISL) questionnaire were analysed. Data from reported weight and height at birth and at one year were used to calculate BMI. Rapid body mass index (BMI) gain was defined by the difference in BMI superior to 1.0z and excessive by the difference superior to 2.0z.
ResultsRapid BMI gain was found in 45.8% infants and excessive in 24.4%. Boys showed a significantly higher BMI gain than girls. Girls with rapid BMI gain showed a significantly higher prevalence of hospitalisation for wheezing (8.8% vs. 6.4%; aOR: 1.4, 95%CI: 1.1–1.8), severe wheezing (18.1% vs. 15.0%; aOR: 1.3, 95%CI: 1.0–1.5) and medical diagnosis of asthma (7.5% vs. 5.7%; aOR: 1.3, 95%CI: 1.0–1.7). Girls with excessive BMI gain also had a significantly higher prevalence of hospitalisation for wheezing (9.8% vs. 6.7%; aOR: 1.5, 95%CI: 1.1–2.0) and severe wheezing (18.9% vs. 15.5%; aOR: 1.3, 95%CI: 1.0–1.6). No significant association was found among boys.
ConclusionsThe majority of the evaluated infants showed BMI gain above expected in the first year of life. Although more commonly found in boys, rapid and excessive BMI gain in the first year of life was significantly related to more severe patterns of wheezing in infancy among girls.
Respiratory diseases are a significant health problem among infants and are responsible for high morbidity and mortality, and also for a large number of unscheduled medical visits and hospitalisations.1 Prospective cohort studies have shown that the majority of wheezing infants have transient symptoms unrelated to asthma.2,3 Infants with severe or recurrent wheezing, however, do not rarely grow up with persistent symptoms and pulmonary impairment.4
According to the ISAAC (International Study of Asthma and Allergies in Childhood) study, asthma is a highly prevalent disease in Brazil, affecting almost a quarter of school-aged children and 20% of adolescents.5 Despite the challenges in establishing a reliable diagnosis, asthma must be considered as one of the leading diagnosis possibilities in infants with recurrent wheezing. It has been estimated that over a third of asthmatics are already symptomatic in the first year of life and that early manifestations are a marker of asthma severity.6
The study EISL (Estudio Internacional de Sibilancias en Lactantes) evaluated the epidemiological profile of wheezing and asthma among infants in several centres in Latin America and Europe employing a validated and standardised instrument.1,7,8 The Brazilian data from this study were obtained from seven state capitals evaluating data from almost 13,000 infants and clearly show the huge impact of wheezing among local infants. Approximately 50% of the surveyed infants had at least one episode of wheezing in the first year of life, over 25% had recurrent wheezing, and 10% had been already diagnosed as asthmatic.1
Several risk factors have been described for recurrent wheezing and asthma in infants, such as male gender, positive family history of asthma, atopic dermatitis and maternal smoking during pregnancy, whereas breastfeeding is one of the few protective factors.9 Rapid weight gain has been recently associated with a higher incidence of wheezing, asthma, and respiratory symptoms in preschool and school-aged children.10–14 This association has not been fully studied for respiratory outcomes in the first years of life.
Asthma and wheezing tend to have different characteristics between genders. Male gender is classically associated with a higher incidence and severity of wheezing and asthma in infancy and childhood and this pattern changes after puberty, when women have a higher incidence of asthma.15 Female gender is also associated with a specific phenotype of severe asthma in adults, mainly non-atopic and related to obesity.16,17
The purpose of this study was to evaluate possible associations and sex differences between body mass index (BMI) changes in the first year of life with the prevalence and severity of wheezing and asthma among infants from six Brazilian state capitals who participated in the EISL study.
MethodsAnswers to the standardised EISL questionnaire obtained in six Brazilian state capitals (São Paulo, Recife, Curitiba, Belo Horizonte, Belém and Cuiabá) were analysed. Details from the EISL study were published elsewhere.1,8 In summary, the standardised written EISL questionnaire was applied to parents or guardians of infants between 12 and 15 months of age who attended health centres for routine immunisation. Local Ethics Committee approval was obtained for all centres as well as signed consent forms from all parents or guardians.
The following data from the EISL questionnaire were analysed: wheezing in the first year of life; recurrent wheezing (three or more episodes) in the first year of life; visit to the emergency department due to wheezing; hospitalisation due to wheezing; medical diagnosis of asthma. Severe wheezing was defined by the presence of recurrent wheezing associated with hospitalisation or with more than six episodes of wheezing in the first year of life.
Duration of breastfeeding and the reported weight and length at birth and at one year of age were also recorded. Weight and length were used to calculate BMI z scores (zBMI) as well as length-for-age and weight-for-age z scores18 employing the WHO Anthro 3.0.1 program.19 Rapid zBMI gain was arbitrarily defined by the difference in zBMI superior to 1.0z and excessive zBMI gain by the difference superior to 2.0z. Infants born weighing less than 2500g or over 4000g were excluded from the study.
Data obtained from the EISL questionnaire were coded in a standard database in Microsoft Excel 2007 and statistically analysed using the SPSS for Windows – Version 18.0. Association between zBMI gain and wheezing and asthma outcomes was tested employing logistic regression after adjusting for breastfeeding (more than four months). Due to the non-parametric distribution of zBMI, the Mann–Whitney test was used to compare numeric variables and Spearman to study correlations. The rejection level of the null hypothesis was 5%.
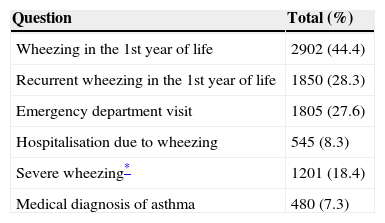
ResultsFrom the 7579 complete questionnaires, 668 were excluded due to weight of birth under 2500g, 273 due to weight of birth over 4000g, and 97 due to inconsistent data. A total of 6541 questionnaires remained for analysis, 3323 (50.8%) of which were from boys. Wheezing in the first year of life was reported in 2902 (44.4%) infants. Prevalence of positive answers to the EISL questionnaire is shown in Table 1.
Affirmative answers to the EISL questionnaire regarding asthma and wheezing (N=6541).
| Question | Total (%) |
|---|---|
| Wheezing in the 1st year of life | 2902 (44.4) |
| Recurrent wheezing in the 1st year of life | 1850 (28.3) |
| Emergency department visit | 1805 (27.6) |
| Hospitalisation due to wheezing | 545 (8.3) |
| Severe wheezing* | 1201 (18.4) |
| Medical diagnosis of asthma | 480 (7.3) |
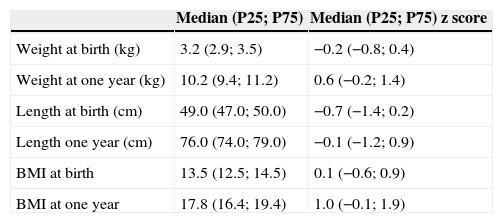
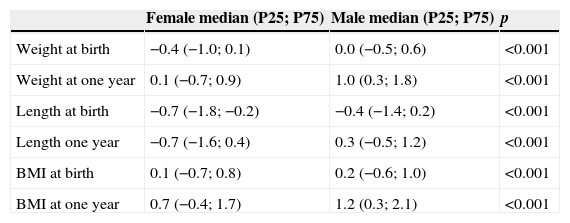
Anthropometric data are shown in Table 2. Girls had significantly lower values than boys for all anthropometric parameters (Table 3). According to WHO classification, at one year 239 (3.7%) infants were malnourished; 3128 (47.8%) were eutrophic; 1624 (24.8%) were at risk of being overweight; 980 (15.0%) were overweight; and 570 (8.7%) were obese. The median change in zBMI in the first year of life was 0.8 (P25: −0.4; P75: 2.0) z score, and was significantly lower among girls than boys (median [P25; P75]: 0.6 [−0.7; 1.9] z score versus 1.0 [−0.2; 2.1] z score, respectively; p<0.001).
Anthropometric data from the included infants (N=6541).
| Median (P25; P75) | Median (P25; P75) z score | |
|---|---|---|
| Weight at birth (kg) | 3.2 (2.9; 3.5) | −0.2 (−0.8; 0.4) |
| Weight at one year (kg) | 10.2 (9.4; 11.2) | 0.6 (−0.2; 1.4) |
| Length at birth (cm) | 49.0 (47.0; 50.0) | −0.7 (−1.4; 0.2) |
| Length one year (cm) | 76.0 (74.0; 79.0) | −0.1 (−1.2; 0.9) |
| BMI at birth | 13.5 (12.5; 14.5) | 0.1 (−0.6; 0.9) |
| BMI at one year | 17.8 (16.4; 19.4) | 1.0 (−0.1; 1.9) |
Anthropometric data in z score discriminated by gender (N=6541).
| Female median (P25; P75) | Male median (P25; P75) | p | |
|---|---|---|---|
| Weight at birth | −0.4 (−1.0; 0.1) | 0.0 (−0.5; 0.6) | <0.001 |
| Weight at one year | 0.1 (−0.7; 0.9) | 1.0 (0.3; 1.8) | <0.001 |
| Length at birth | −0.7 (−1.8; −0.2) | −0.4 (−1.4; 0.2) | <0.001 |
| Length one year | −0.7 (−1.6; 0.4) | 0.3 (−0.5; 1.2) | <0.001 |
| BMI at birth | 0.1 (−0.7; 0.8) | 0.2 (−0.6; 1.0) | <0.001 |
| BMI at one year | 0.7 (−0.4; 1.7) | 1.2 (0.3; 2.1) | <0.001 |
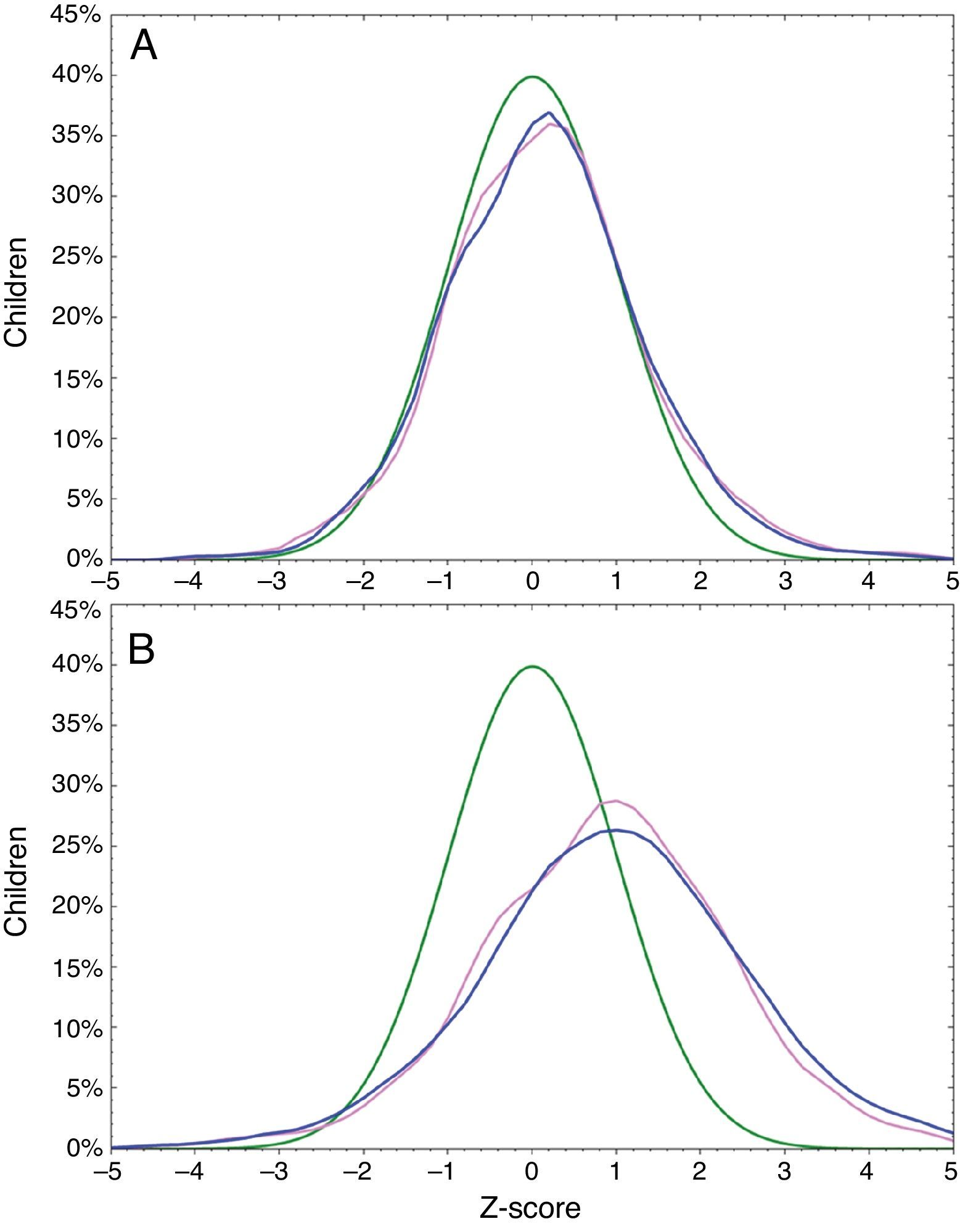
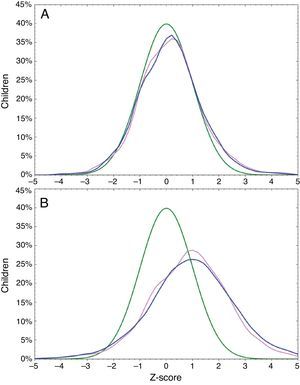
Rapid zBMI gain was observed in 2999 (45.8%) infants; 1342 (41.7%) girls; and 1657 (49.9%) boys. Excessive zBMI gain was found in 1596 (24.4%) infants; 724 (22.5%) girls; and 872 (26.2%) boys. Median of breastfeeding was 5.0 months (P25: 3.0 months; P75: 6.0 months) and 4537 (69.4%) infants were breastfed for at least four months. Overlap of zBMI at birth and at one year of age is shown in Fig. 1.
Rapid and excessive zBMI gain was significantly associated with lower prevalence of breastfeeding in total group and among both girls and boys. Rapid zBMI gain was significantly associated with higher prevalence of severe wheezing and hospitalisation due to wheezing (Table 4). When the group was separated by gender, no significant association was found among boys, but among girls rapid zBMI gain was significantly associated with higher prevalence of severe wheezing, hospitalisation due to wheezing and medical diagnosis of asthma (Table 4). In a similar way, excessive zBMI gain was associated with higher report of severe wheezing and hospitalisation due to wheezing among girls, with no association among boys (Table 5).
Significant associations between rapid BMI gain (>1 z score) in the first year of life and the questions from the EISL questionnaire in the total group (N=6541) and among girls (N=3218).
| Total group – rapid BMI gain (N=2999) | ||||
|---|---|---|---|---|
| Yes (%) | No (%) | p | aOR (95%CI) | |
| Hospitalisation due to wheezing | 280 (9.3) | 265 (7.5) | 0.02 | 1.2 (1.0–1.5) |
| Severe wheezing* | 588 (19.6) | 613 (17.3) | 0.04 | 1.1 (1.0–1.3) |
| Female infants – rapid BMI gain (N=1342) | ||||
|---|---|---|---|---|
| Yes (%) | No (%) | p | aOR (95%CI) | |
| Hospitalisation due to wheezing | 118 (8.8) | 120 (6.4) | 0.02 | 1.4 (1.1–1.8) |
| Severe wheezing* | 243 (18.1) | 281 (15.0) | 0.03 | 1.3 (1.0–1.5) |
| Medical diagnosis of asthma | 100 (7.5) | 106 (5.7) | 0.04 | 1.3 (1.0–1.7) |
aOR, odds ratio adjusted for breastfeeding; CI, confidence interval.
Significant associations between excessive BMI gain (>2 z scores) in the first year of life and the questions from the EISL questionnaire in the total group (N=6541) and among girls (N=3218).
| Total group – excessive BMI gain (N=1596) | ||||
|---|---|---|---|---|
| Yes (%) | No (%) | p | aOR (95%CI) | |
| Hospitalisation due to wheezing | 159 (10.0) | 386 (7.8) | 0.01 | 1.3 (1.1–1.6) |
| Female infants – excessive BMI gain (N=724) | ||||
|---|---|---|---|---|
| Yes (%) | No (%) | p | aOR (95%CI) | |
| Hospitalisation due to wheezing | 71 (9.8) | 167 (6.7) | 0.008 | 1.5 (1.1–2.0) |
| Severe wheezing* | 137 (18.9) | 387 (15.5) | 0.04 | 1.3 (1.0–1.6) |
aOR, odds ratio adjusted for breastfeeding; CI, confidence interval.
The change in zBMI in the first year of life was significantly higher among those with severe wheezing (median [P25; P75]: 1.0 [−0.3; 2.1] z score versus 0.8 [−0.5; 2.0] z score, respectively; p=0.03). There was a significant and negative correlation between zBMI changes in the first year of life and zBMI at birth (r=−0.53; p<0.001).
DiscussionIn this study we have evaluated changes in BMI in the first year of life from a large number of Brazilian infants, randomly selected from all regions of the country. It is widely known that the pattern of weight gain during childhood in Brazil and in many other countries has shown a rapid change in recent years with a worrying increase in obesity.20–22 This observation was confirmed in our study, where almost half of the infants were classified as obese, overweight or at risk of being overweight at one year of age. According to a national survey, 6% of Brazilian infants are classified as obese in the first year of life,20 a proportion of obesity quite similar to that found in our study (8.7%). This high proportion of obesity is probably due to inadequate feeding patterns such as premature interruption of breastfeeding20 and frequent use of whole cow's milk in the first year of life.23 This high proportion of obesity and overweight noted at one year of age does not seem to be related to birth weight, as can be observed in Fig. 1.
Rapid weight gain at an early age has been considered one of the most important factors associated with several chronic non-infectious diseases. Strong evidence relates rapid weight gain in the first years of life to childhood and adult obesity.24,25 On the other hand, obesity is being increasingly related to asthma, with a similar pattern of prevalence growth in the last few decades.26 The mechanisms involved in the interconnections between these two diseases are not fully understood and involve genetic, hormonal, immunological and mechanical factors.27 Inflammation is also present in both asthma and obesity and has been described as a central pathophysiological mechanism in the association with the two diseases.26 Obesity is known to be a risk factor for the development of asthma,28 severe asthma,29 greater lung function impairment26 and low response to asthma treatment.27
Recently, the results from several cohorts regarding the association between weight gain and wheezing and asthma have been published. Van der Voort et al. followed a cohort of 5125 Dutch newborn babies up to the age of four and found that rapid weight gain in the first three months of life was significantly associated with higher risk of respiratory symptoms such as wheezing and cough.12 In another European cohort, 9806 German newborn babies were followed up until the age of 10 and rapid weight gain in the first two years of life was significantly associated with a higher prevalence of medical diagnosis of asthma.13 Similar results were found in the study of Rzehak et al. where data from eight European birth cohorts were combined with more than 12,000 children followed up until six years of age.14 Children with rapid BMI gain in the first two years of life had a higher risk for incident asthma up to the age of six than children with a normal BMI gain (hazard ratio of 1.3).14
The majority of available data regarding the relation between infant weight gain and asthma/wheezing are looked at the medical diagnosis of asthma at school age as a primary outcome and very little is known about the relationship between weight gain and early respiratory outcomes. However, the associations between weight gain in infancy and asthma and/or wheezing in the first years of life seem to be concentrated in outcomes related to severity, such as recurrent wheezing and hospitalisations. In a prospective cohort of 932 children, Taveras et al. found that infants with higher weight-for-length z scores at six months had greater risk of recurrent wheezing by age three.30 In this study, each 1-unit increment in six-month weight-for-length z score was associated with 1.46 greater odds of recurrent wheezing. In a three-year follow-up in a clinical trial involving 197 infants with high risk for asthma, those with rapid weight gain in infancy had higher use of oral steroids and more frequent visits to the emergency department due to wheezing.31
Mechanisms involved in the association between early life weight gain and asthma and/or respiratory symptoms in young children are still not understood. In one study, infants with rapid weight gain in the first year of life had lower postnatal pulmonary function growth.32 It has already been documented that infants with excessive weight gain have higher blood levels of insulin-like growth factor (IGF)-I, somatomedin with an important role in growth and adipogenesis in early life.33 IGF-I is also known to be an important mediator of airway inflammation and remodelling.34,35 In a murine asthma model, administration of IGF-I neutralising antibody inhibited OVA inhalation elevation of airway resistance and inflammation.36
Surprisingly, sex differences are still randomly reported in cohort studies that evaluated the relationship between weight gain and asthma in children. In our study, the impact of weight gain on respiratory outcomes in the first year of life, particularly those related to wheezing severity, was found exclusively among girls. It is already known that the association between obesity and/or adiposity with asthma is stronger among women.37,38 It was once estimated that 28% of asthma developing in women after age nine was due to overweight.38 Female hormones are usually pointed to as being responsible for this association in teenagers and adult women.37,38 Possible mechanisms involved in the association between weight gain and severe wheezing in female infants are not known. In an interesting clinical trial infants were randomly selected to receive infant formulas with different protein levels during the first year of life and were compared to a breastfed group.39 Infants with higher protein intake had higher weight gain and higher IGF-I levels. Gender differences were observed in the relationship between IGF-I levels and protein intake, with a stronger positive association among female infants. If IGF-I is indeed involved in the association between early weight gain and asthma and/or respiratory symptoms in infants, as speculated, this association would theoretically be stronger among females, as we have found.
In summary, we have found that the majority of the evaluated infants showed an above expected BMI gain in the first year of life. Although more commonly found in boys, rapid and excessive BMI gain in the first year of life was significantly associated with more severe patterns of wheezing in infancy among girls.
Conflict of interestThe authors have no conflict of interest to declare.
Ethical disclosuresPatients’ data protectionConfidentiality of Data. The authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Protection of human subjects and animals in researchThe authors declare that no experiments were performed on humans or animals for this investigation.
Brazilian EISL Group: Elaine X. Prestes (Universidade Estadual do Pará), Herberto Chong Neto (Universidade Federal do Paraná), Nelson Rosário Filho (Universidade Federal do Paraná), Ana Carolina Dela Bianca (Universidade Federal de Pernambuco), Carolina Aranda (Universidade Federal de São Paulo), Décio Medeiros (Universidade Federal de Pernambuco), Emanuel Sarinho (Universidade Federal de Pernambuco), Lilian S. Moraes (Universidade Federal do Mato Grosso), Maria Jussara Fernandes-Pontes (Universidade Federal de Minas Gerais), Paulo Camargos (Universidade Federal de Minas Gerais).