Co-morbid allergic rhinitis (AR) and asthma has not been studied in Caribbean countries where there is a high prevalence of childhood asthma.
MethodsUsing the International Primary Care Airways Group (IPAG) guidelines to determine AR, care-givers of 393 (response rate=100%) children attending asthma clinics in selected public sector health facilities in Trinidad, West Indies, were interviewed.
ResultsChildren (393) were between 2–17 years and included 239 (60.8%) boys and 154 (39.2%) girls. As many as 53.9% of children sampled (95% CI 45.9–55.8) suffered from AR. Children exposed to household smoking were nearly twice as likely to have AR (p<0.0041, OR=1.9, CI 1.22–2.88). Significantly (p<0.01) more asthmatics with AR (154, 58.6%) visited Accident and Emergency (A&E) in the past 12 months. The odds of visiting A&E at least once in the past 12 months for asthmatics with AR were 1.75 (95% CI 1.15–2.68). The average frequency of A&E visits was higher in children who also suffered from AR (1.75 vs 1.36, p<0.04). Age was negatively correlated (−0.21, p<0.005) with exacerbation frequency for asthmatics without AR suggesting A&E visits are independent of age in co-morbid disease. More children with AR (>60%) suffer day and night symptoms (p<0.001), and miss school (59.8%) (p<0.03) at least once a week (p<0.002) than asthmatics without AR (OR=1.5, 95% CI=1.03–2.30).
ConclusionsAR is prevalent in 53.9% of Trinidadian children with asthma. The burden of co-morbid disease in asthmatic children is associated with increased likelihood of asthma-related A&E visits, day and night symptoms and absence from school.
Asthma and allergic rhinitis (AR) are chronic inflammatory airway disorders, which often occur concomitantly as manifestations of a united airway disease in an anatomic continuum with structural and functional differences.1 The World Health Organization initiative on Allergic Rhinitis and its Impact on Asthma (ARIA) has developed evidence-based guidelines to manage AR and highlights its impact on asthma.2 ARIA reports that about 80% of patients with asthma also have AR which worsens asthma, increases the risk of hospitalisation and asthma exacerbations. From epidemiological studies as many as 78% of patients with asthma experience nasal symptoms and as many as 38% of patients with AR also suffer with asthma.3 AR recognised as a risk factor for the development of asthma4 usually precedes asthma.5
In the majority of asthmatic children AR manifests early in life.6 Co-morbid AR exacerbates asthma symptoms, increases asthma-related hospitalisations and emergency department (ED) visits, GP visits, and overall asthma-related drug costs in children with asthma.6,7 Evidence of lung inflammation in rhinitis patients can be detected even when they do not have overt asthma symptoms. The ARIA guidelines recommend patients with persistent rhinitis be evaluated for asthma and those with persistent asthma be assessed for rhinitis.2 The ARIA guidelines, culminating current understanding of the shared pathophysiology, health outcomes and costs, recommend a ‘one airway’ approach in disease management, addressing treatment of the upper and lower airways.2,4
Although the clinical association of AR and asthma has been recognised, results observed in developing countries may differ from those in Western populations. Much of the data on childhood asthma in Caribbean countries has come from Puerto Rico,8,9 with few reports from the Anglophone Caribbean.10,11 Despite the strong association between asthma and AR, as far as these authors are aware very little is known about the prevalence of AR in the region, in children who also suffer from asthma. We aimed to obtain data on prevalence of AR in a population of asthmatic children attending public sector asthma clinics in Trinidad and its association with asthma-related clinical burden. The IPAG (International Primary Care Airways Group) guidelines12,13 informed on the definition of AR.
Materials and methodsEthical approvalThe study was approved by the Ethics Committee of the Faculty of Medical Sciences, The University of the West Indies. Trained researchers administered a pilot-tested questionnaire in an interview format to patients or their care-givers after receiving informed consent.
ProcedureThe study employed a cross-sectional design to determine the prevalence of AR based on clinical history in children attending an asthma clinic at selected health care facilities in Trinidad between June and August 2008. The target population included children between 3–17 years of age with a doctor's diagnosis of asthma, attending public health facilities in Trinidad. Children were recruited as they presented at the Arima Health Centre where patients from the east and north attend the San Fernando General Hospital in the south, and the Couva and Chaguanas health facilities in central Trinidad. These clinics provide care for the majority of children with asthma on the island.
AssessmentAsthmatic children were categorised into two groups: those with AR and those without. Children meeting the criteria for AR as set out in the IPAG guidelines12 according to which those children who suffered with watery runny nose and one or more of the additional listed symptoms ever suffered in the past 12 months were considered to have AR. The additional symptoms of AR in the guidelines include nasal obstruction, nasal itching, sneezing especially violent and in bouts and conjunctivitis (red, itchy eyes). A pilot-tested questionnaire was administered to care-givers by trained researchers. Care-givers were asked if in the past 12 months children made unscheduled visits to A&E (outside of the scheduled follow-up care) or needed to be relieved from wheezing by nebulisation. They were also asked if children suffered symptoms of asthma during the day, or woke up at night with symptoms. In addition to wheezing, specific symptoms of asthma enquired about were cough, chest tightness and breathlessness. Absence from school was considered important for this study if it was due to symptoms of asthma.
Statistical analysisThe calculated sample size required to estimate prevalence within 5% at the 95% confidence level was 393 subjects. Confidence intervals (CI) were based on 95% level and differences in rates were tested based on Normal distribution. To assess for associations between AR/asthma and smoking, symptom prevalence, visits to A&E, day and night-time disturbances the Pearson's chi square test was used; odds ratios were determined where relevant. The ANOVA was used to compare mean number of unscheduled visits to A&E by AR and non-AR subjects.
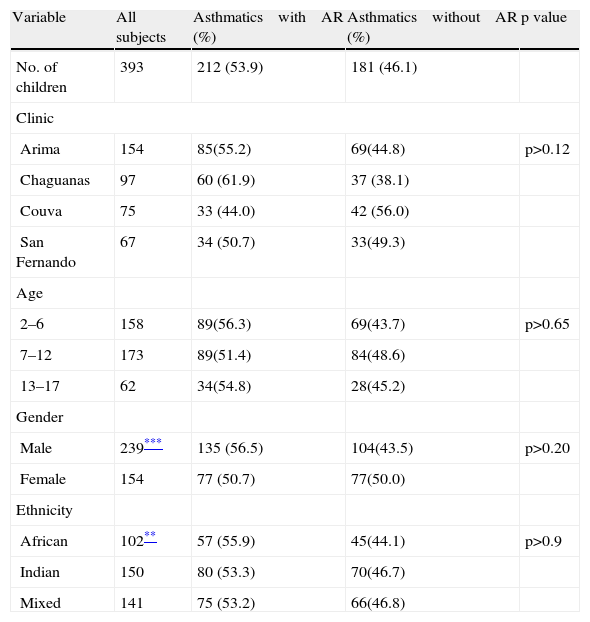
ResultsDemographicsNearly forty percent of the patients were from Arima (154, 39.2%); a quarter came from Chaguanas (97, 24.7%); and the rest were from Couva (75, 19.1%); and San Fernando (67, 17.0%) (Table 1). The difference between the proportions of boys and girls in the target population was consistent between the two disease groups (p>0.20), with higher proportions of boys than girls in the two disease groups. Thus, among the subjects examined, the percentage of boys (239, 60.8%) is significantly higher than 50% (p<0.0001). Age and gender distribution of children did not differ among clinics at the 5% level. The mean ages of boys and girls were 7.99 (SD 3.57) and 8.27 years (SD 4.15), respectively. Subjects of African descent appear to be under-represented (102, 26.0%) compared with children (150, 38.1%) of East Indian origin and those of mixed ancestry (141, 35.9%) (p<0.002). Nearly half of the children (197, 50.1%) lived close to a main road or highway. Others lived close to industrial plants (76,19.3%) and bush fires or burning sites (64, 16.3%), and few lived proximal to landfills (12, 3.4%), quarries (25, 6.4%), or other environments (26, 6.6%). Some subjects were resident in an environment with potentially more than one risk factor. None of these environmental factors appeared to put children at risk for AR. Just over half of all children (211, 53.7%) kept pets and the proportion of asthmatics with AR who kept pets was higher and significant only at the 6% level. Care-givers of a third of children (131, 33.3%) said they were exposed to cigarette smoke at home. Asthmatic children who were exposed to smoking in the household were twice as likely to have AR as those who were not exposed to household smoking (p<0.0041, OR=1.9 CI=1.22–2.88).
General characteristics of asthmatics with and without allergic rhinitis (%)
| Variable | All subjects | Asthmatics with AR (%) | Asthmatics without AR (%) | p value |
| No. of children | 393 | 212 (53.9) | 181 (46.1) | |
| Clinic | ||||
| Arima | 154 | 85(55.2) | 69(44.8) | p>0.12 |
| Chaguanas | 97 | 60 (61.9) | 37 (38.1) | |
| Couva | 75 | 33 (44.0) | 42 (56.0) | |
| San Fernando | 67 | 34 (50.7) | 33(49.3) | |
| Age | ||||
| 2–6 | 158 | 89(56.3) | 69(43.7) | p>0.65 |
| 7–12 | 173 | 89(51.4) | 84(48.6) | |
| 13–17 | 62 | 34(54.8) | 28(45.2) | |
| Gender | ||||
| Male | 239*** | 135 (56.5) | 104(43.5) | p>0.20 |
| Female | 154 | 77 (50.7) | 77(50.0) | |
| Ethnicity | ||||
| African | 102** | 57 (55.9) | 45(44.1) | p>0.9 |
| Indian | 150 | 80 (53.3) | 70(46.7) | |
| Mixed | 141 | 75 (53.2) | 66(46.8) | |
All the 393 children who were approached consented to participate (response rate 100%). Two hundred and twelve (53.9%) (95% CI 45.9–55.8) of these asthmatic children also suffered from AR (Table 1).
Visits to accident and emergencySignificantly (p<0.01) more children with AR (154, 58.6%) visited A&E in the previous 12 months than those without AR (109, 41.4%). The odds of visiting the A&E department at least once for children with AR were 1.75, (95% CI 1.15–2.68). Asthmatics with AR made more visits on average than those without AR (1.75 vs 1.36, p<0.04). The least squares mean difference for visits made by children with and without AR was 0.39(±0.185). Asthmatic children who did not suffer with AR demonstrated a negative correlation (−0.21), (p<0.005) between age and the frequency of A&E visits. On the other hand there did not appear to be any correlation between age and A&E visits in asthmatics with AR (p>0.73), suggesting that in the presence of co-morbid AR and asthma, the risk of unscheduled A&E visits is independent of age.
Impact on well being-symptom disturbancesSubjects reported various symptoms associated with asthma and AR. However, a significantly higher proportion of asthmatic children with AR consistently experienced all symptoms with the exception of coughing (Table 2). Proportionally more children who suffered with AR reported chest tightness (65.2%) and breathlessness (59.3%), (p<0.001 and p<0.02 respectively than children who had asthma alone.
Symptoms of asthma and allergic rhinitis which children suffered
| Symptoms of asthma or allergic rhinitis which children suffered | All children n=393(%) | Asthmatics with AR (%) n=212 | Asthmatics without AR(%) n=181 |
| Symptoms of Asthma | |||
| Cough | 120 (30.5) | 67 (55.8) | 53 (44.2) |
| Chest Pain (Tightness) | 164 (41.7) | 107(65.2) | 57(34.8)*** |
| Breathlessness | 182 (46.3) | 108(59.3) | 74 (40.7)** |
| Wheezing | 295 (75.0) | 167(56.6) | 128 (43.4)* |
| Symptoms of Allergic Rhinitis | |||
| Runny nose | 212(53.9) | 212 (100.0) | 0(0.0) |
| Sneezing in violent bouts | 206(52.4) | 149(72.3) | 57(27.7)*** |
| Nasal obstruction | 172(43.8) | 121(70.4) | 51(29.9)*** |
| Itchy, red eyes | 186(47.3) | 140(75.3) | 46(24.7)*** |
| Itchy nose | 184(46.8) | 129 (70.1) | 55(28.9)*** |
AR=Allergic Rhinitis.
Subjects with AR are 1.6 (95% CI 1.10–2.45) times more likely to suffer at least one day time disturbance than those who suffer from asthma alone.
Allergic rhinitis also influences night-time symptoms in asthmatic children (p<0.001). Among children who reported night-time symptoms, a higher proportion (60.0%) had AR, and the frequency of night-time symptoms was also dependent on co-morbid AR with asthma (p<0.0001). A higher proportion of children who had co-morbid asthma and AR (60.1%) experienced two or more symptoms per week. Children with co-morbid disease are 2.6 times (95% CI 1.65–4.18) more likely to suffer at least one night-time disturbance than those with asthma alone.
Impact on well-being missing schoolAllergic rhinitis affects attendance at school in asthmatic children (p<0.03). Proportionally fewer children (40.2%) who suffered from asthma alone reported missing school due to illness. Asthmatics with AR are 1.5 (95% CI 1.03–2.30) times more likely to miss school than children with asthma alone. The number of times children missed school is also dependent on AR (p<0.026). A higher proportion of children with co-morbid disease (63.0%) missed school once or twice in a week (p<0.002).
DiscussionProportionately half of the children with asthma attending primary health care facilities in Trinidad, suffer with AR. In Trinidad and Tobago the 12-month prevalence of wheeze is 25% in school children aged 12 to 15 years,11 but information on the prevalence of symptoms of AR in these children is not available. We found that as many as 53.9% of children diagnosed with asthma also suffer from AR using symptom criteria in the IPAG guidelines, in the absence of the common cold and/or the ‘flu’. This has important implications for the management of the asthmatic child in Trinidad and prompts physicians to be alert to the presence of AR in paediatric asthma. Family doctors (primary care physicians, general practitioners) as the first point of contact for patients, play a major role in diagnosing and managing AR. Support for primary care practice has come from a joint expert panel of the World Organization of Family Doctors (WONCA), the International Primary Care Airways Group (IPAG) and the International Primary Care Respiratory Group (IPCRG), by extracting the globally accepted, evidence-based recommendations from the Allergic Rhinitis and its Impact on Asthma (ARIA) initiative in a brief reference guide on the management of AR.14 The ARIA reports that up to 80% of both adults and children who are afflicted with asthma suffer from AR.2 Lasmar et al. found a point prevalence of 74.6% of AR in Brazilian asthmatic children between 3–17 years.15 The differences between our findings and other studies are possibly due to differences in the target population and/or standards adopted. For example Lasmar et al. studied only those children with moderate to severe persistent asthma, and used the ARIA guidelines to diagnose AR based on the single or combined presence of itchy nose and/or orophayrnx; serous or seromucus rhinorrhoea; sneezing; itchy eyes; and nasal congestion. We used the IPAG criteria to inform on the diagnosis of AR where watery runny nose is associated with at least one of the other symptoms from sneezing in violent bouts; nasal obstruction; nasal itching; red itching and/or watery eyes; and itchy throat, as the study was conducted in primary health care facilities. The reported prevalence rates of co-existent AR and asthma in children from Brazil, Turkey and Greece, range widely from 30% to 90%4,16–19. The findings of Hamouda et al. who reported AR was prevalent in 58.7% of French asthmatic children between 3–18 years20 strengthen our results. These studies like ours were done in ambulatory patients, and underscore the need for population-based studies in children in Trinidad and the wider Caribbean.
Conventional diagnostic techniques are insufficient to discriminate in preschool children between true asthma and the transient wheeze of viral infections. Moreover, the majority of the children with recurrent asthma-like symptoms are symptom free at 6 years,21 and do not have asthma. Children in the present study were diagnosed based on clinical features consistent with asthma, and repeated episodes of wheezing, coughing or shortness of breath that respond to bronchodilators.
The significantly higher prevalence of asthma in boys has been observed in studies from Trinidad22, Spain,23 and Sao Paulo.24 Asthmatic children who were exposed to smoking in the household were twice as likely to suffer with co-morbid AR as those who had asthma alone, suggesting exposure to tobacco smoke may facilitate the development of AR and also worsen asthma. A previous report from Trinidad and Tobago found smoke was strongly associated with symptoms of asthma and rhinitis in primary school children.25 Allergic rhinitis appears to impact negatively on children with asthma, and asthma-related visits to A&E are likely to be increased by 1.75 times. Asthmatic children who suffer from AR should be closely monitored for disease exacerbations and encouraged to diligently take controller medication on guideline-based recommendations. Our results are supported by findings from a database of 2,961 Norwegian asthmatic children with AR who had a 1.72 times greater hazard of hospital re-admissions than those without AR.26 In a general practice database of 9522 asthmatic children in the United Kingdom, those with co-morbid AR experienced more GP visits (4.4 vs 3.4) and had more hospitalisations for asthma (1.4% vs 0.5%) in a 12-month follow-up period than did children with asthma alone.6 Kang et al. studied asthma-related claims in 319,714 chronic asthmatic children in Korea and found that those with AR had more outpatient visits (1.14 times), more emergency department visits (1.30 times) and more hospitalisations (1.49 times) than children without AR.27 Coexistent asthma and AR identifies a high-risk group of children in Trinidad who should be targeted for continual care with regular follow-up visits.
More asthmatic children with AR experienced wheeze, breathlessness and chest tightness of asthma than children who suffered with asthma alone. It appears that in the presence of AR asthmatic children suffer more with symptoms of asthma, pointing towards the need to control AR. Allergic rhinitis that is inadequately controlled in asthmatic patients increases exacerbations of asthma and results in poorer symptom control with the consequent increases in utilisation of medical resources.28 Three-quarters of paediatric patients in an international survey of co-morbid AR in asthmatics reported worsening of asthma symptoms when AR symptoms got worse.7 Our findings are encouraged by the ARIA recommendations that all patients with asthma be checked for AR and vice versa.2 It is important to detect and treat co-morbid AR and asthma because the condition is associated with increased likelihood of asthma-related emergency visits, absence from school and greater frequency of day and night-time symptoms. Evidence suggests that co-morbid AR is a marker for more difficult to control asthma and worsened asthma outcomes. Thomas reported asthma outcomes can be improved by following a combined therapeutic approach in co-morbid AR and asthma rather than targeting each condition separately.28 Montelukast induced improvements in AR which were associated with better asthma control in asthmatic children.29 We recommend asthmatic children be investigated for the presence of AR and offered treatment for both diseases. Co-morbid AR and asthma deteriorates asthma outcomes in children who may benefit from a combined therapeutic approach.
The study demonstrated that AR interrupts the life of asthmatic children and restricts their activities. Compared with children who suffered from asthma alone, those with concomitant AR and asthma experienced more frequent day and night-time symptom disturbances in a week, and missed more school days in any one week. Sleep, leisure and concentration at school are adversely affected in asthmatic children with AR. Parents of children with asthma and AR from four countries each in the Asia-Pacific region and Europe said children did not enjoy social events (51%) or concentrate at school (73%).7 In fact, parents of 30-35% of these children believed that asthma and AR were disruptive to their general well-being.
The study was limited by the three-month period in which data was collected; therefore children who express symptoms of AR outside those months may not be represented. The diagnosis of AR was based on history and awareness of symptoms. Although the operational definition of AR used in this study has not been validated in epidemiological studies Bousquet et al.14 have discussed it in the pocket reference for the management of AR. Hypersensitivity skin tests for specifc IgE mediated allergic reactions, serum measurement of allergen-specifc IgE and nasal challenge tests which could yield confirmatory evidence of allergic disease were not carried out.
ConclusionsFifty-three point nine percent of children with asthma attending primary health care facilities in Trinidad suffer with AR. Co-morbid AR worsens the clinical burden of asthma and is associated with more visits to A&E; day and night-time symptoms; and absences from school.
Conflict of interestThe authors have no conflict of interest to declare.
Dr. Celia Poon King critiqued the protocol and Professor Terence Seemungal reviewed the manuscript. Administrators at the South West Regional Health Authority, St George East County, and the Chaguanas Health Facility gave permission to conduct the study at the respective facilities.