Present in several types of food, bioactive amines are described as organic bases of low molecular weight, which constitute a potential health risk. An awareness of amine levels in foods today is therefore important in relation to food safety and patient care. This review aims to emphasise the need to unify the information on the content of biogenic amines in foods and prevent patients’ misunderstanding.
MethodsSelective literature search for relevant publications in PubMed and other scientific data bases combined with further data from the World Wide Web on histamine and other amines content in foods.
ResultsAvailable reference sources do not reflect a homogeneous consensus, and the variation between foods makes it impossible for dieticians to accurately estimate amines content to correctly advise patients.
ConclusionsTo achieve the goal of collecting reliable information, all methods and tools used in analytical studies should be standardised and information exposed to patients should be verified.
Bioactive amines are described as organic bases of low molecular weight produced by the metabolism of plants, animals and microorganisms. They have vasoactive, psychoactive and toxicological characteristics and constitute a potential health risk. The concentration of amines formed in foods depends on the type of microorganisms present, the action of decarboxylase enzymes produced by microorganisms on specific amino acids and favourable conditions for enzymatic activity. The presence of these chemical metabolites has been suggested as a quality indicator in routine analyses for food production and marketing monitoring.1
Biogenic amines are present in low concentrations or are not detected in fresh food (below 10μg/g); however, in food of animal origin such as fish, meat, eggs, cheese and fermented foods, they can be present in high concentration (above 50μg/g) able to induce chemical poisoning.2
The most important biogenic amines found in food are: histamine, tyramine, tryptamine, phenethylamine and cadaverine, the synthesis of which occurs by decarboxylation of the precursor amino acids histidine, tyrosine, tryptophan, phenylalanine and lysine, respectively.3
Based on the mean content of the most toxic biogenic amines (histamine and tyramine), the food safety relevance of the considered food categories can be ranked in the following decreasing order – for histamine: ‘dried anchovies’, ‘fish sauce’, ‘fermented vegetables’, ‘cheese’, ‘other fish and fish products’ and ‘fermented sausages’; and for tyramine: ‘fermented sausages’, ‘fish sauce’, ‘cheese’, ‘fermented fish’ and ‘fermented vegetables’. Based on the consumer exposure to the most toxic biogenic amines, the food safety relevance of the considered food categories can be ranked in the following decreasing order – for histamine: ‘other fish and fish products’, ‘fermented sausages’, ‘cheese’, ‘fish sauces’ and ‘fermented vegetables’; and for tyramine: ‘beer’, ‘cheese’, ‘fermented sausages’, ‘fermented fish meat’ and ‘preserved meat’.4
Histamine is also contained in mast cells and basophils, and its biological effects are usually seen only when it is released in large amounts in the course of allergic and other reactions.5
Amines can be formed by transamination of aldehydes and ketones, hydrolysis of nitrogen compounds, thermal decomposition or by decarboxylation of amino acids.6 The decarboxylation process can proceed through two biochemical pathways: decarboxylation through endogenous (naturally occurring) decarboxylase enzymes or by exogenous decomposition through enzymes released by microflora. The production of amines by the exogenous process is considered far more significant.7
According to their biosynthetic pathway, bioactive amines are classified into1:
- 1)
Biogenic: they are formed by bacterial enzymatic decarboxylation of amino acids (histamine, serotonin, tyramine, phenethylamine, tryptamine, putrescine, cadaverine and agmatine).
- 2)
Natural: spermine and spermidine are formed “in situ” in the cells as they are required.
It is noteworthy that since histamine is stored in mast cells, it could be classified as biogenic and natural.
There is a natural mechanism for bioactive amine catabolism. They are oxidised by amine oxidades (monoamine oxidases (MAO) and diamine oxidases (DAO)).8 Amine oxidases are enzymes which catalyse the oxidative deamination of mono-, di- and polyamines with the formation of an aldehyde, ammonia and hydrogen peroxide: RCH2NH2+O2+H2O RCHO+H2O2+NH3.7
The metabolic function of these enzymes is the breakdown of a number of biologically active amines. The enzymes are divided into two separate groups:
The first group is the flavin co-factor dependent enzymes known as monoamine oxidases (MAO).7
The so-called ‘trace amines’ are synthesised in humans from their corresponding amino acids (tyrosine, phenylalanine and tryptophan, respectively) by decarboxylation. Their catabolism is mainly mediated by monoamine oxidase (MAO). Two MAO isozymes exist, A and B, with different locations and substrate specificity. MAO-A predominates in the stomach, intestine and placenta and has polar aromatic amines (as noradrenalin and octopamine) as preferred substrates. MAO-B predominates in the brain and selectively deaminates non-polar aromatic amines (as phenethylamine and dopamine).4
Tyramine is a substrate for either form of MAO, MAO-A is responsible for intestinal metabolism of tyramine, thereby preventing its systemic absorption. Tyramine and phenethylamine are also subjected to N-methylation by N-methyltransferases, generating the sympathetic neurotransmitter, noradrenaline. Tyramine can be further converted into octopamine.9
The second group is the copper amine oxidases or diamine oxidases (DAO), which are found in animal tissue and plasma, plants, yeasts, fungi and bacteria.7
Humans metabolise histidine to urocanic acid through the activity of L-histidine ammonium lyase, to form glutamate and then α-ketoglutarate, which enters the citric acid cycle, or to histamine through the activity of histidine decarboxylase. There are two ways of histamine metabolism in the human body. Nitrogen in the imidazole cycle is methylated by histamine N-methyltransferase at the formation of N-methylhistamine, which is further oxidised by monoamino oxidase to N-methylimidazolylacetic acid. This enzyme is very selective for histamine detoxification and involves S-adenosylmethionine as donor of methyl group. Histamine is oxidised by diamino oxidase to imidazolylacetic acid, which is bound to ribose.5
The biogenic amine content of some foods has been widely studied because of their potential toxicity. Histamine has been implicated as the causative agent in outbreaks of food poisoning where intoxication results from the ingestion of foods containing excessive amounts of histamine. Tyramine and phenethylamine have been identified as the initiators of hypertension during treatment with monoamino oxidase inhibitor drugs and of dietary-induced migraine in susceptible individuals.4
The accumulation of biogenic amines in food depends on the availability of free amino acids and the presence of microorganisms with decarboxylase activity on amino acids.10 Amine formation also depends on food intrinsic and extrinsic parameters such as: temperature and pH, oxygen tension, availability of carbon sources, presence of vitamins, co-enzymes, concentration of free amino acids and fermentable carbohydrates.11
Individual differences in enzyme activities besides varying amine concentrations in food may account for different tolerance levels.12
In general, increased sensitivity against biogenic amines is due to a weakened enzymatic amine degradation caused by genetic or acquired impairment of MAO, DAO, histamine-N methyltransferase (HNMT) function.4
Impairment of DAO activity either due to genetic predisposition, gastrointestinal diseases, or due to medication with DAO inhibitors results in high histamine blood levels, which consequently overload the internal hepatic inactivation system of histamine-N methyltransferase,12 and leads to histamine intolerance causing numerous symptoms mimicking an allergic reaction even after the ingestion of small amounts of histamine tolerated by healthy individuals.13
DAO has been shown to be a secretory protein predominantly synthesised in renal proximal tubular and intestinal epithelial cells and it is stored in plasma membrane associated vesicular structures. Therefore, it has been proposed that DAO might be of primary importance for inactivation and scavenging of extracellular histamine originating from ingested histamine rich food.14
Individuals with respiratory and coronary heart diseases, hypertension problems or vitamin B12 deficiency are at risk because they are sensitive to smaller amounts of amines. People with gastrointestinal problems (gastritis, irritable bowel syndrome, Crohn's disease, gastric and colon ulcers) are also at risk since the activity of the oxidases in their bowels is generally lower than in healthy individuals. People using drugs MAO, DAO and polyamine oxidase (PAO) activity inhibitors may also be affected because they prevent amine catabolism.1
Other diseases, such as mastocytosis and mast cell activation syndrome (MCAS), are also related to histamine, as Mast Cells (MCs) are the main source of histamine in the human intestine.15
Mastocytosis is divided into cutaneous mastocytosis (CM), systemic mastocytosis (SM), and local MC tumours (mastocytoma and MC sarcoma), all of them alterations of the MC.16
MCs are tissue-fixed effector cells of allergic and other inflammatory reactions. Mast cell activation (MCA) occurs in a number of different clinical conditions, including primary mast cell disorders, IgE-dependent allergies and atopic diseases, other hypersensitivity or immunologic reactions, and mastocytosis. MCA can be documented by a substantial increase in the serum tryptase level above baseline. Apart from tryptase, other mediators, may also serve as useful parameters of MCA. These include, among others, histamine (in plasma and urine) and histamine metabolites (in urine).17
When symptoms are recurrent, are accompanied by an increase in mast cell-derived mediators in biological fluids, and are responsive to treatment with mast cell-stabilising or mediator-targeting drugs, a MCAS may be diagnosed.17
Therapy is based on the consequent conduction of a histamine-free diet. A histamine-free diet, if necessary, supported by antihistamines or the substitution of DAO, leads to an improvement of symptoms.13
Alcohol and long-ripened or fermented (and therefore histamine-rich) food, such as aged cheese, cured meat, and yeast products; histamine-rich food, such as spinach or tomatoes; or histamine liberators, such as citrus fruit, should be avoided.13
In addition, the histamine-free diet can be complemented with adjuvant administration of H1 and H2 antagonists and histamine degradation can be supported by the administration of vitamin C and vitamin B-6, which leads to an increase in DAO activity.13
Histamine intolerance sufferers have different thresholds, i.e. tolerance levels, so the next step after completing a successful elimination diet is to establish their threshold level, with the aim of gradually improving it over a period of time.18
These amines do not represent a health hazard unless they are consumed in large quantities or if the natural catabolism mechanism of one or more amines is inhibited. In this case, poisoning occurs and thus it is important to determine amine profile and content in food since these substances can trigger toxicological processes.
We therefore sought to review the actual histamine and other biogenic amines content of a number of food tables and judge the reference source that physicians might consult in advising patients with histamine and other amines intolerance.
MethodsSearch strategyThe present text was a review. It focuses on scientific literature about histamine intolerance and related diseases. Thus, a bibliographic search was made on PubMed data base and other scientific data bases, such as Scielo, Embase, Cochrane, Clinicaltrials.gov and Scopus, using the following MeSH terms and keywords: “histamine”[MeSH Terms] OR “histamine”[All Fields] AND intolerance[All Fields]; bioactive[All Fields] AND (“amines”[MeSH Terms] OR “amines”[All Fields]); amine oxidase (copper-containing)”[MeSH Terms] OR (“amine”[All Fields] AND “oxidase”[All Fields] AND” (copper-containing)”[All Fields]) OR “amine oxidase (copper-containing)”[All Fields] OR (“diamine”[All Fields] AND “oxidase”[All Fields]) OR “diamine oxidase”[All Fields]; mast cells”[MeSH Terms] OR (“mast”[All Fields] AND “cells”[All Fields]) OR “mast cells”[All Fields] OR (“mast”[All Fields] AND “cell”[All Fields]) OR “mast cell”[All Fields]. This research strategy was adapted to each search engine.
The studies undertaken on histamine were reviewed in January 2016. The search was done by two independent researchers who subsequently corroborated the results found.
Inclusion/exclusion criteriaThe following types of studies were included: food composition tables, food analytical studies and laboratory analysis, reviews, table compilations and proposals of content in food for the general population.
The exclusion criteria were as follows: duplicated studies or non-related with the topic, controversial biomarkers to get and support results or conclusions, opinions, expert's opinions, letters, conference papers and editorial papers.
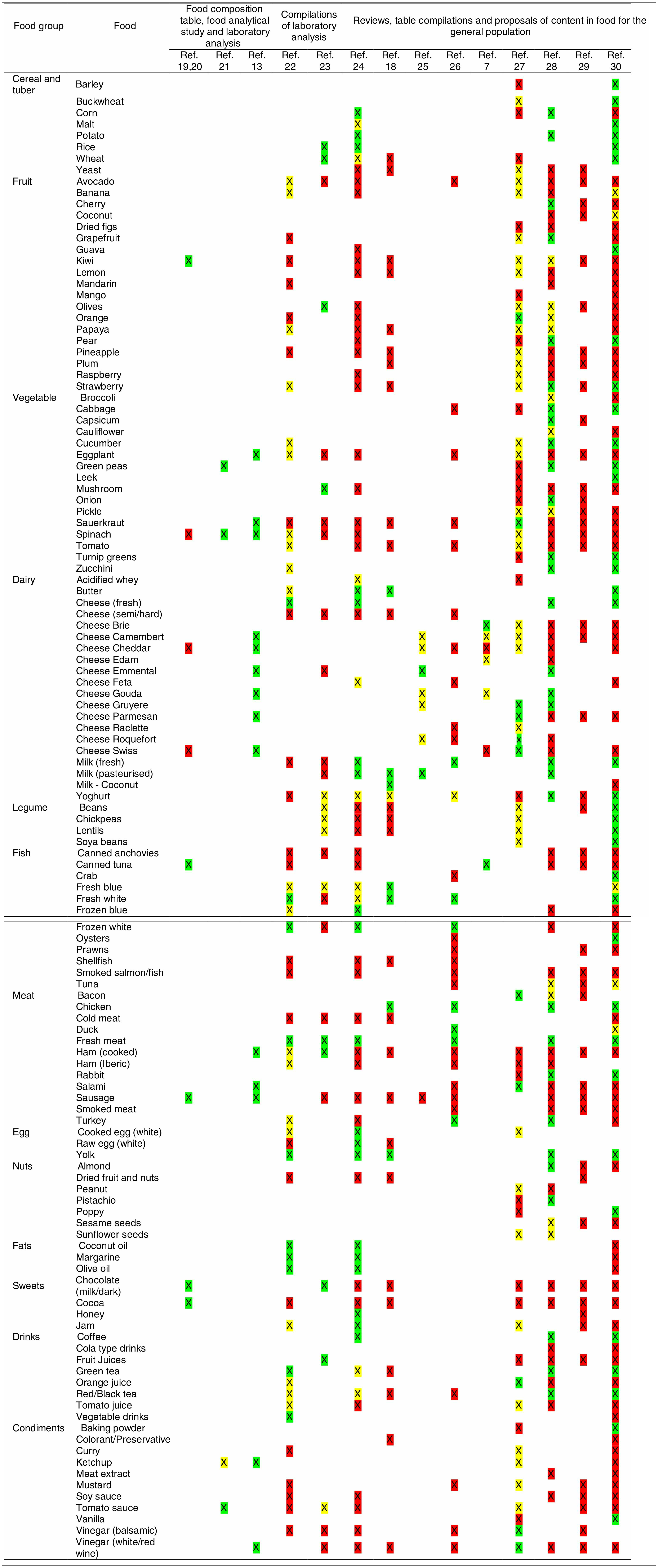
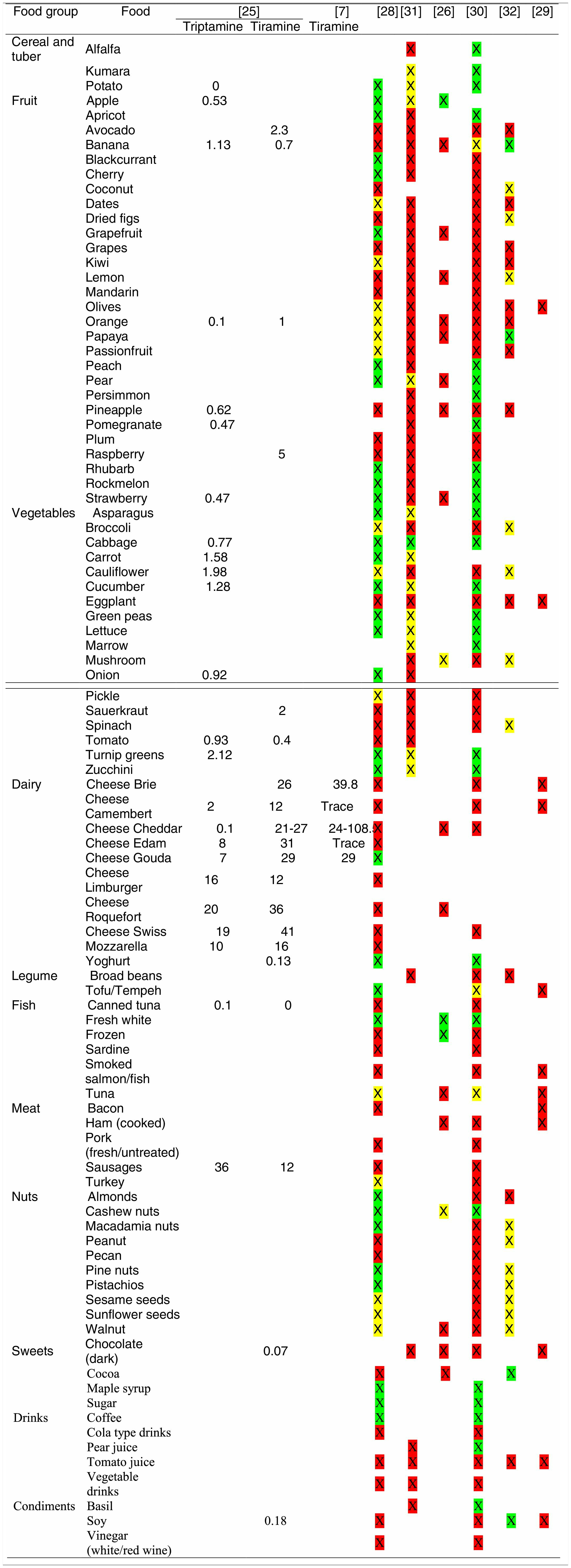
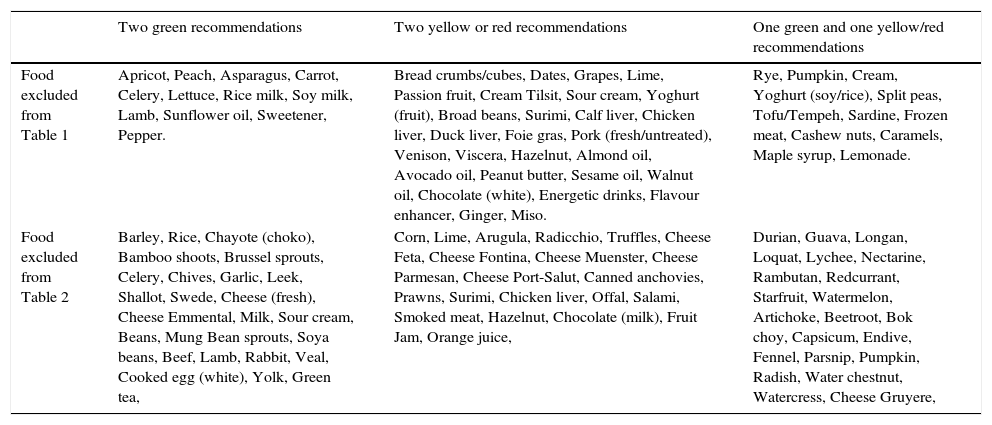
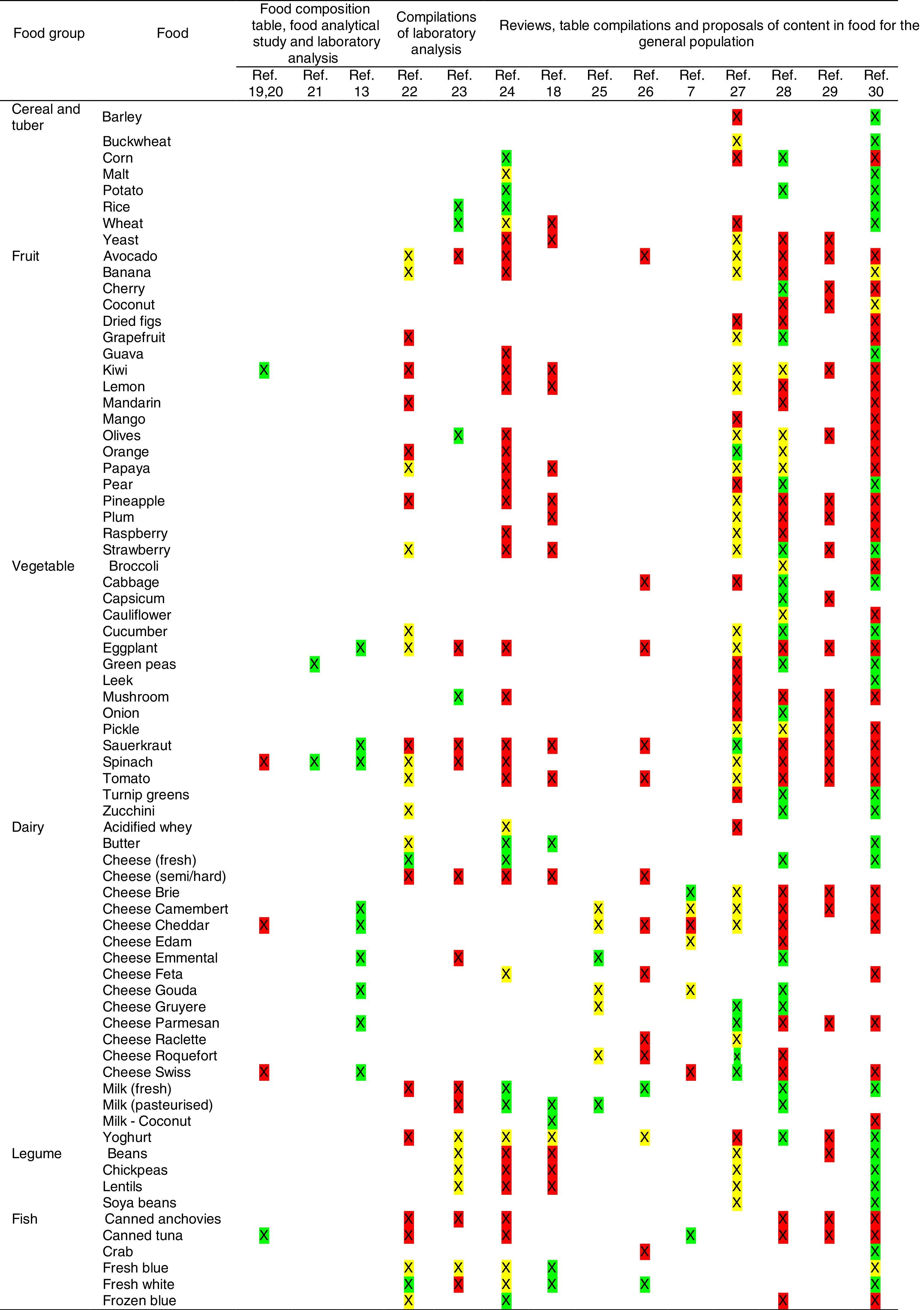
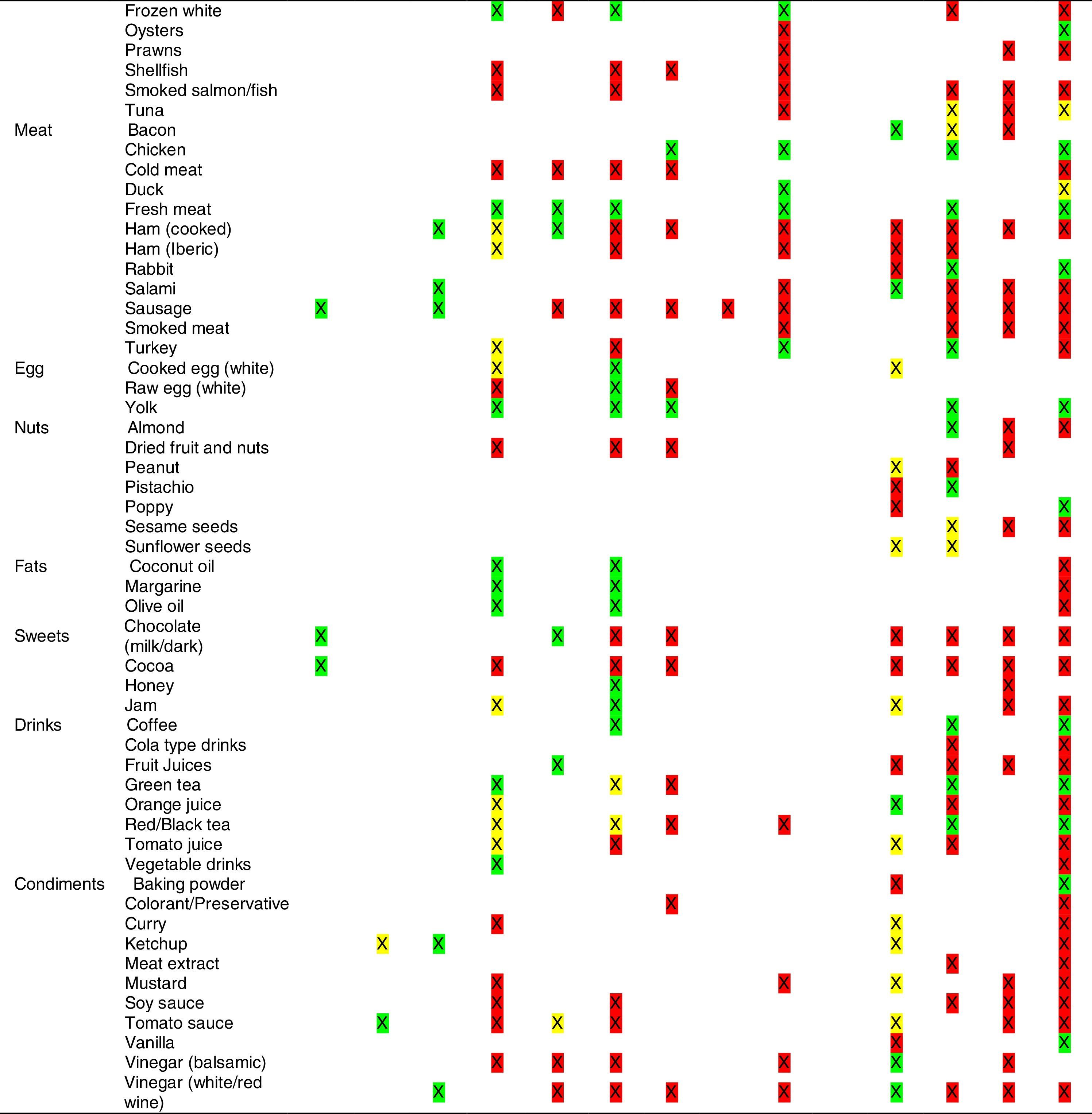
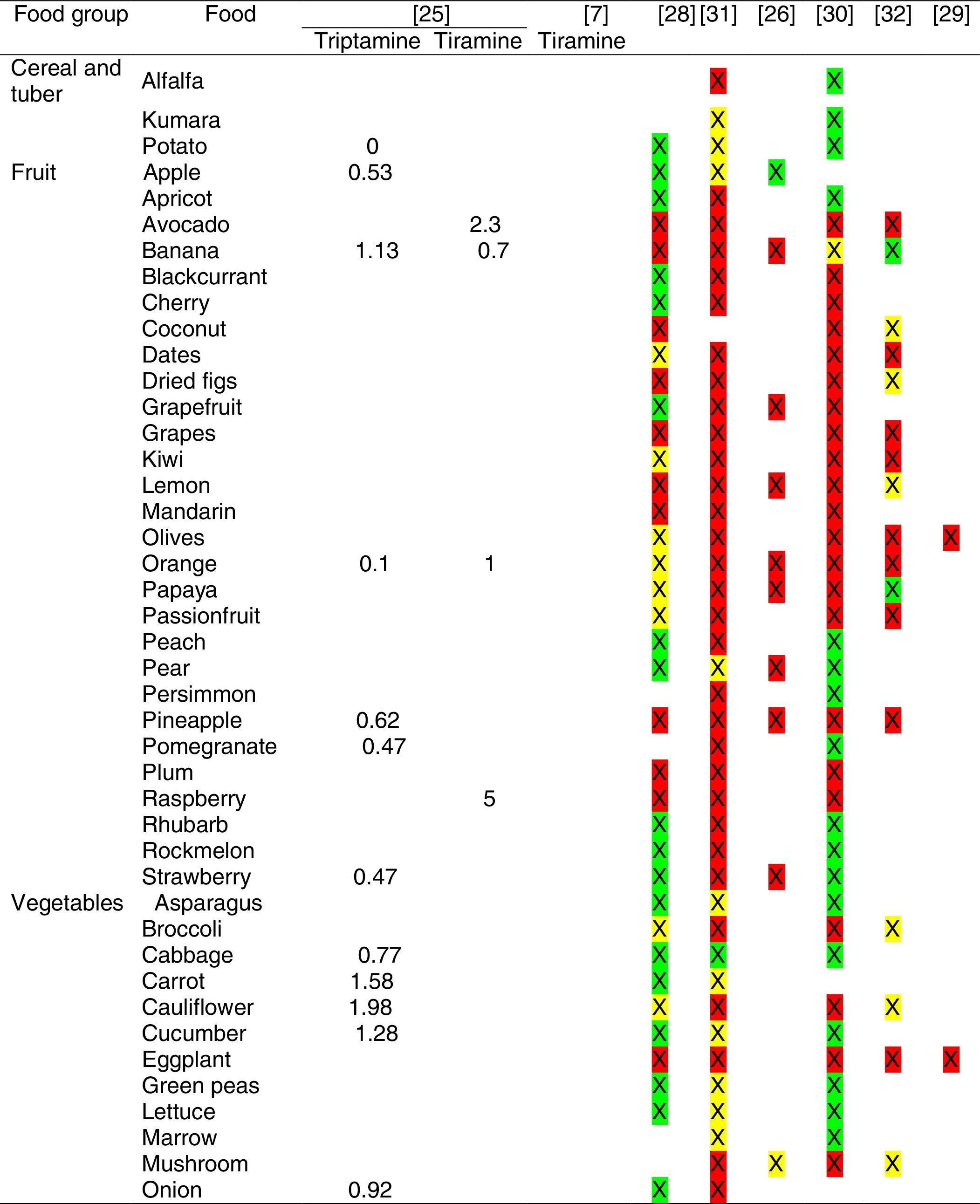
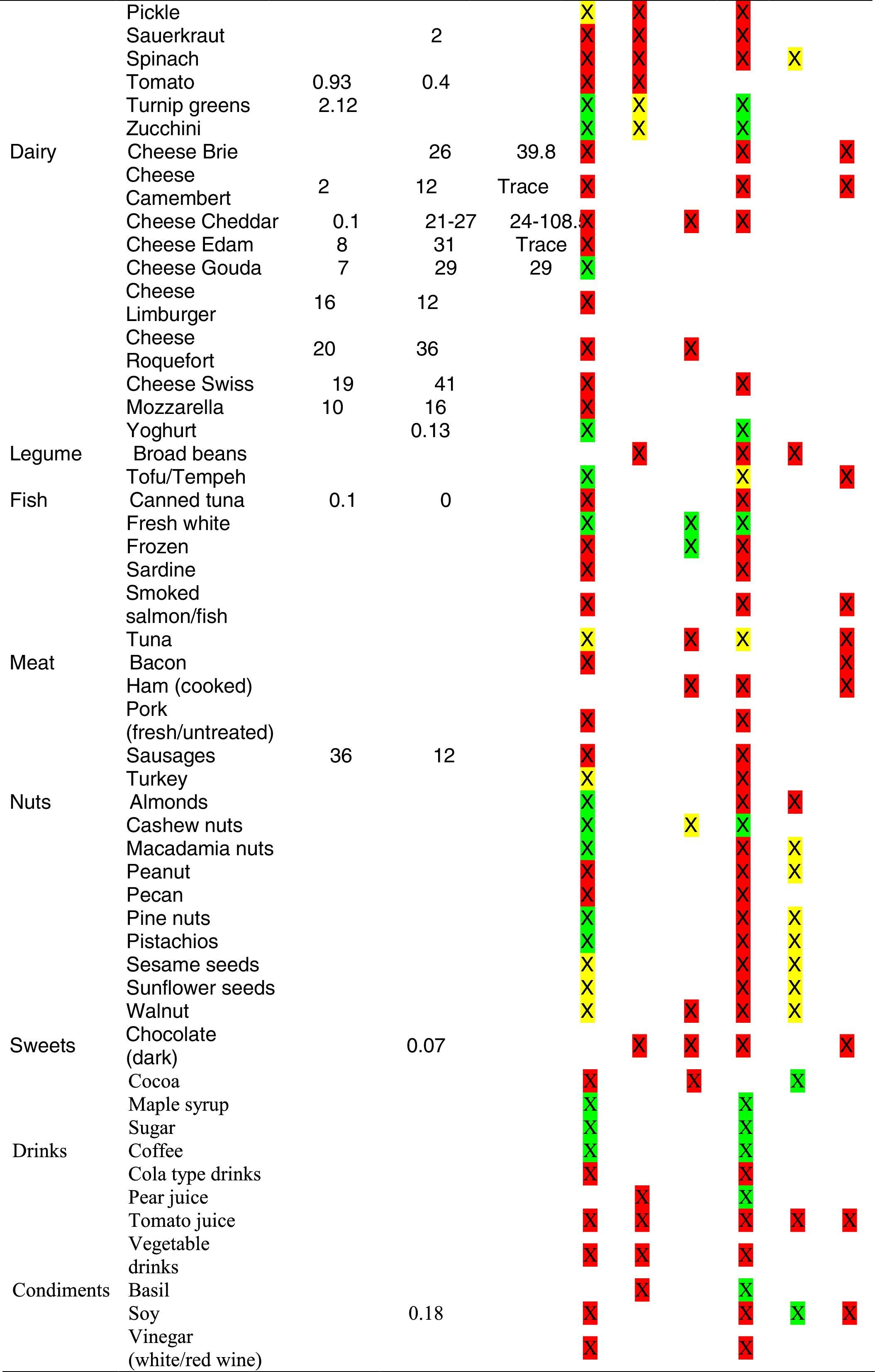
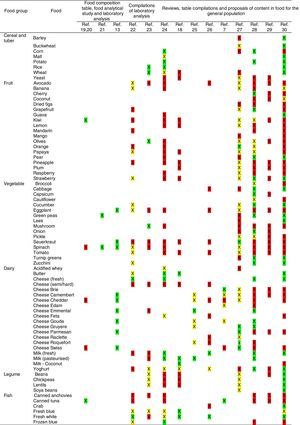
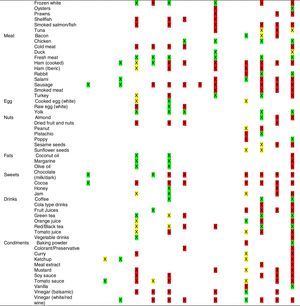
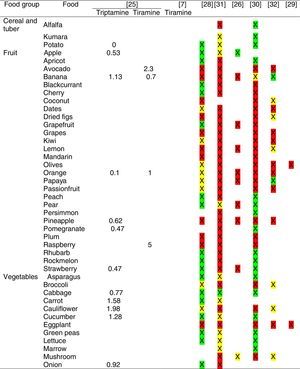
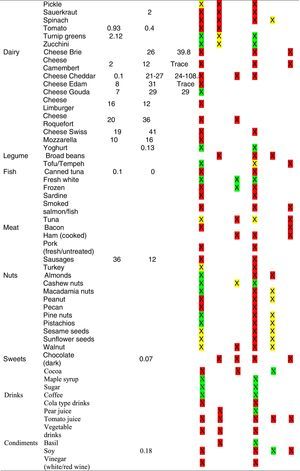
Tables were divided in three. Table 1 shows a comparison between reviews,19–32 table compilations and proposals of histamine content in food for the general population and food analytical studies and laboratory analysis. Table 2 is similar to Table 1, but shows other amines content in food and Table 3 collects those foods that were excluded from Tables 1 and 2 under the following criteria: less common foods or only 2 out of 14 references included the food in their list, both had the same colour recommendation or on the contrary had opposite colour recommendations.
Foods excluded from Tables 1 and 2.
| Two green recommendations | Two yellow or red recommendations | One green and one yellow/red recommendations | |
|---|---|---|---|
| Food excluded from Table 1 | Apricot, Peach, Asparagus, Carrot, Celery, Lettuce, Rice milk, Soy milk, Lamb, Sunflower oil, Sweetener, Pepper. | Bread crumbs/cubes, Dates, Grapes, Lime, Passion fruit, Cream Tilsit, Sour cream, Yoghurt (fruit), Broad beans, Surimi, Calf liver, Chicken liver, Duck liver, Foie gras, Pork (fresh/untreated), Venison, Viscera, Hazelnut, Almond oil, Avocado oil, Peanut butter, Sesame oil, Walnut oil, Chocolate (white), Energetic drinks, Flavour enhancer, Ginger, Miso. | Rye, Pumpkin, Cream, Yoghurt (soy/rice), Split peas, Tofu/Tempeh, Sardine, Frozen meat, Cashew nuts, Caramels, Maple syrup, Lemonade. |
| Food excluded from Table 2 | Barley, Rice, Chayote (choko), Bamboo shoots, Brussel sprouts, Celery, Chives, Garlic, Leek, Shallot, Swede, Cheese (fresh), Cheese Emmental, Milk, Sour cream, Beans, Mung Bean sprouts, Soya beans, Beef, Lamb, Rabbit, Veal, Cooked egg (white), Yolk, Green tea, | Corn, Lime, Arugula, Radicchio, Truffles, Cheese Feta, Cheese Fontina, Cheese Muenster, Cheese Parmesan, Cheese Port-Salut, Canned anchovies, Prawns, Surimi, Chicken liver, Offal, Salami, Smoked meat, Hazelnut, Chocolate (milk), Fruit Jam, Orange juice, | Durian, Guava, Longan, Loquat, Lychee, Nectarine, Rambutan, Redcurrant, Starfruit, Watermelon, Artichoke, Beetroot, Bok choy, Capsicum, Endive, Fennel, Parsnip, Pumpkin, Radish, Water chestnut, Watercress, Cheese Gruyere, |
Low content <5mg/kg (green); moderate content 5–20mg/kg (yellow); and high content >20mg/kg (red).
For tables that provided the amount of histamine in mg/kg per food, low content was considered to be <5mg/kg (green); moderate content 5–20mg/kg (yellow); and >20mg/kg as high content (red).
ResultsSearch resultsA total of 23 references were retrieved in this review. After reading all the sources of information we were led to the exclusion of nine of them for not following the proposed criteria or being duplicated. Thus, only 14 studies were considered legible for review and have been included after a double revision. All studies reviewed have shown low-medium evidence as a whole.
DiscussionWe found that histamine and other biogenic amines are present in a vast majority of foods. Available reference sources do not reflect a homogeneous consensus, and the variation between foods makes it impossible for dieticians to accurately estimate amines content to correctly advise patients.33
It has been shown how in the reviews, compilation tables and proposals of foods content for the overall population, three different recommendations (in response to high, moderate or low histamine content) were found for the same food. This was the case of strawberries, blue cheese, cooked ham, yoghourt, frozen blue fish or green tea.
It can also be seen, when comparing those compilation tables with laboratory analytical studies, how histamine content recommendations for the same food can be opposed (very high vs. very low content). Foods such as kiwi, spinach, canned tuna, sausages, cocoa and tomato sauce showed this contrast.
It may also occur that even laboratory analytical studies show opposite results, as with spinach, Cheddar and Swiss cheese and ketchup.
Even the European Rapid Alert system for Food and Feed database reports concentration ranges in different sub-samples of the same sample e.g. 50–500mg/kg, 102–180mg/kg, 27–152mg/kg, of 147mg/kg, below 5–208 and below 10–1000mg/kg (for fish samples).4
Data from outbreaks show big variation in concentrations leading to adverse effects in the consumer. These uncertainties may lead either to an underestimation of the adverse effect to occur in sensitive people or to an overestimation of the risk in healthy people due to biogenic amines, since only the risk assessment of the individual biogenic amines is feasible at the moment.4
That situation can lead patients to assume erroneous or insufficiently verified information, and generates incomplete or ineffective treatments. This becomes disturbing when two-thirds of those using the internet to find health information claim it has some impact on their healthcare decisions,34 and when searchers assure the internet information they obtain is, in most cases, trustworthy.35
The majority of health-related Internet searches by patients are for specific medical conditions.36 Many concerns have been raised about the quality of online consumer health information, and the possibility that poor information has detrimental effects on health. A systematic review of 79 studies investigating the quality of online health information revealed that the methodology and rigour of these studies varies widely, as do the findings.37 The information is often incomplete and sometimes inaccurate, although good quality material can be found.
Therefore, instructing patients with histamine intolerance, DAO deficit and mast cell diseases to limit their intake of histamine and other amines containing products would seem to be a reasonable recommendation. However, following such a recommendation will be challenging for several reasons. First, simply knowing that a product contains histamine or other amines does not allow an accurate estimate of its content. Second, ingredient lists are generally not present on fast food items, making it difficult to identify the presence of amines. Third, amine-free products may require more effort to identify.33
To achieve the goal of collecting reliable information, all the methods and tools should be standardised.38 Standardisation is a sine qua non of information pooling. However, scientific, and other constraints make it difficult to impose uniform procedures across studies.39 In this case, each assessed nutrient must be defined in the same way, the units of measurement must be comparable, and the methods used to assess nutrient value must be the same or comparable.38 It is important to recognise that it is not always necessary to use precisely the same methods and tools for data collection in order to achieve valid data integration across studies. Rather, what is crucial is that the information conveyed by each data set is ‘inferentially equivalent’.39
Nutrient values in the food composition databases must be updated frequently and new foods and recipes need to be added promptly so that the results are precise and accurate also over time.38 We recommend that manufacturers analyse their products for amines content and make these data available for incorporation into nutrient databases. Food manufacturers should also lower amines content of popular products. We also recommend that policymakers mandate that amines content of foods be included on the nutrition facts label. These actions by manufacturers and policymakers will help patients limit their amines intake, will help providers to better instruct patients, and will help researchers to accurately assess dietary intake.33
ConclusionA valid risk assessment requires data on exposure, and thus on the contents of contaminants in foods. However, these data are highly variable and may significantly differ even within narrowly confined regions. In this regard, more attention should be paid to the preparation, extension and maintenance of food composition databases.
Further studies are necessary to establish safety limits for bioactive amines in food, and the intolerance they may cause.
Ethical disclosuresConfidentiality of dataThe authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
Protection of human subjects and animals in researchThe authors declare that no experiments were performed on humans or animals for this investigation.
Conflicts of interest and source of fundingThe authors have disclosed that they have no significant relationships with, or financial interest in, any commercial companies pertaining to this article.