INTRODUCTION
Factors involved in pregnancy loss (PL) due to abnormal fetomaternal tolerance are poorly understood. PL has been shown to be associated in a proportion of cases with the antiphospholipid syndrome (APS) or with aberrant natural killer cell activity which indicate that the etiology may involve some form of immune dysfunction1.
A novel role for complement as an effector in the pathway leading to PL has been suggested2. However, the clinical significance of complement is unclear in patients with unexplained PL. Low levels of complement 3 (C3) and/or complement 4 (C4) are reported to be associated with the APS3. In the mouse model, activation of maternal C3 through the alternative pathway has been shown enough to mediate defects in placenta formation and subsequent PL which is independent of the presence of B cells and autoantibodies2.
Identification of complement activation as a mediator of PL and definition of the complement components necessary to trigger such injury is likely to lead to a better understanding of its pathogenesis and to new and improved treatments.
In the present study we determined the prevalence of hypocomplementemia in a cohort of women with recurrent PL, with special attention given to women without circulating autoantibodies.
MATERIALS AND METHODS
In a retrospective transversal study we investigated 201 women with recurrent PL in the Immunology Department at the University Hospital Gregorio Marañón. The patients were admitted to the hospital or followed at the outpatient clinic for other Departments (Genetics, Infertility or Obstetrics), with a history of recurrent spontaneous PL. Recurrent PL was defined by the loss of two or more consecutive pregnancies of non-viable foetuses1.
In order to exclude other causes of recurrent PL, paternal chromosomes, uterine anomalies and endocrine pathologies had been evaluated before by the referral departments.
To investigate possible causes of hypocomplementemia, histories of the patients were carefully reviewed by the same immunologist for any clinical criteria of autoimmune diseases, hepatopathy and concomitant chronic infections known to be associated with complement consumption. Clinical manifestations of APS were considered according to recent Sydney consensus document4. An underlying autoimmune disease was considered when the patients met specific criteria according to the American College of Rheumatology criteria5-6. If the patient's AST and/or ALT level rose above 1.25 fold above the upper level of normal even once, they were classified as having hepatopathy. In all cases the serum was recovered from the blood samples by sedimentation at 1500 Xg and was thawed and analyzed by nephelometry (Beckman-Coulter). Total haemolytic activity of the complement system (CH100) was investigated by radial immunodiffusion test. In this study, low levels of complement factors C3, C4, Factor B (FB) and CH100 were define as lower than the 5th percentile obtained in 30 age-matched healthy women who had normal pregnancies but no PL (healthy controls): < 72, < 14, < 21 mg/dl and < 26 CH100 Units/ml, respectively. As pregnancy may have an effect on complement levels we included women who were parous as controls7. Control women were drawn from a normal population after completion of a questionnaire. Immunological studies of patients and controls were performed at least three months after PL or successful pregnancy, respectively.
Other immunological parameters that were collected included IgG and IgM anticardiolipin antibodies (ACA, measured by ELISA), IgG and IgM anti-β2-glycoprotein-I antibodies (anti-β2-GP-I, measured by ELISA), antinuclear antibodies (ANA, measured by indirect immunofluorescence), anti-DNA antibodies (anti-DNA, measured by ELISA), anti-thyroid antibodies (measured by ELISA) and immunoglobulin (Ig) G, IgA and IgM levels (measured by nephelometry, Beckman-Coulter). Lupus anticoagulant was performed by coagulimetric tests in the Haematology Department. In this study we defined high title ANA when the title was ≥ 1/160. Hypergammaglobulinemia was defined as the presence of serum concentrations of immunoglobulins > 95th percentile observed in healthy controls (IgG > 1403 mg/dl, IgA > 415 mg/dl, IgM > 211 mg/dl).
RESULTS
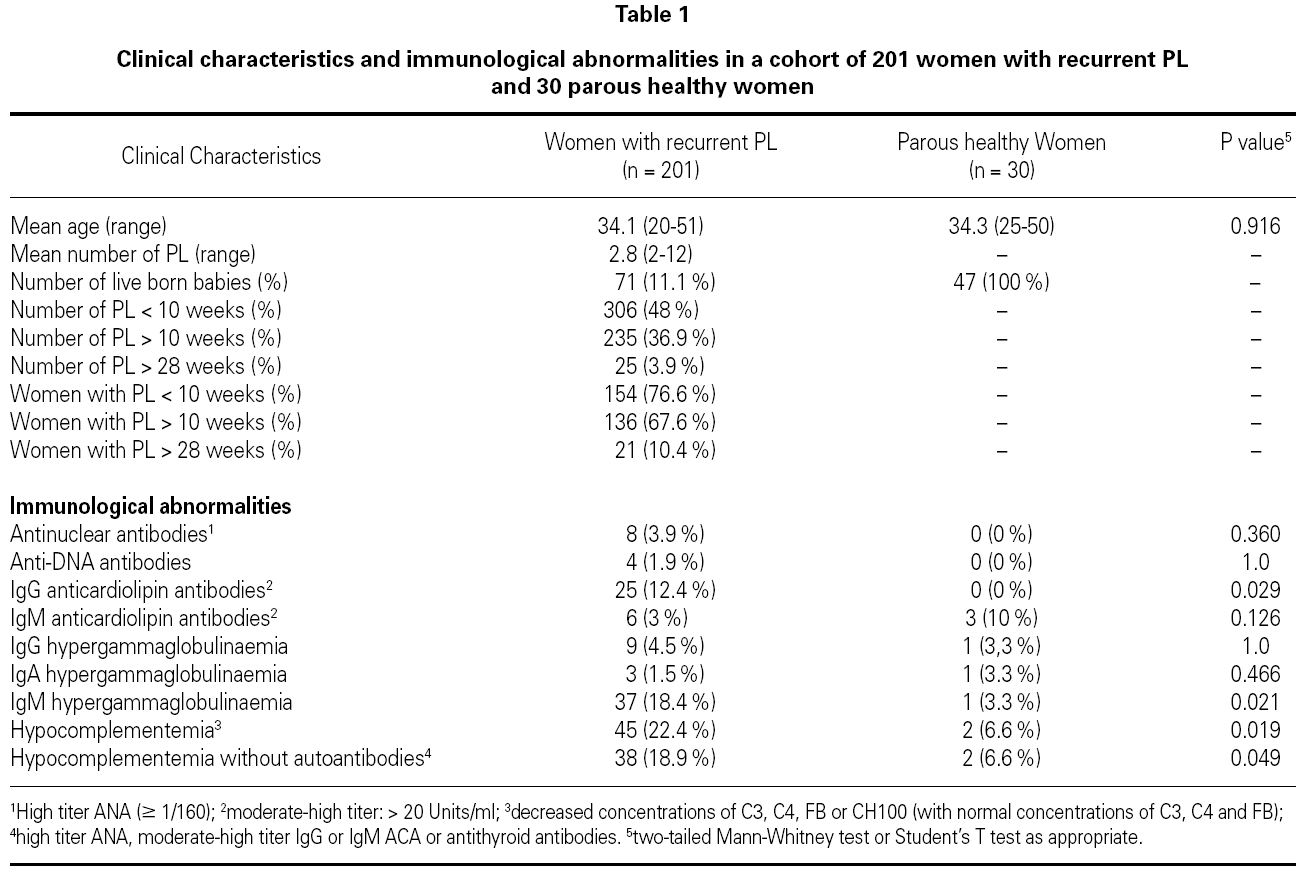
Clinical and immunological characteristics of the 201 women with recurrent PL and of 30 parous healthy women are shown in table 1.
The prevalence of hypocomplementemia [low levels of C3, C4, FB or CH100 (with normal concentrations of C3, C4 and FB)] was significantly higher in women with recurrent PL (45 out of 201 patients, 22,4 %) in comparison with controls (2 out of 30 women, 6.6 %; 2-sided Fisher's exact test, p = 0.019).
C3 hypocomplementemia was observed in 13 (6.5 %) women with recurrent PL. The mean concentration of C3 in this group was 61 mg/dl (range: 22-71 mg/dl). At these group of women with low C3 complement, 10 patients had no circulating autoantibodies [high titer ANA (> 1/160), moderate-high titer IgG or IgM ACA (> 20 Units/ml) or antithyroid antibodies) and 3 had autoantibodies [IgG-ACA (n = 2) and IgM-ACA (n = 1)]. Low C3 was observed in 1 healthy woman.
C4 hypocomplementemia was detected in 19 patients (9.45 %, mean concentration of C4: 12 mg/dl, range: 8-13 mg/dl). 18 patients had no positive autoantibodies and one patient showed a positive lupus anticoagulant. Low C4 levels in combination with low CH100 activity was observed in one healthy control.
Factor B hypocomplementemia occurred in 13 patients (6,5 %, mean concentration of FB: 17 mg/dl, range: 12-20 mg/dl). Only one patient had positive autoantibodies (IgG-ACA).
Low CH100 with normal nephelometric values of C3, C4 and Factor B was observed in 7 patients (3,5 %, medium level of CH100: 22 Units/ml, range: 18-23 Units/ml). Five of these patients did not have circulating autoantibodies. One woman had low CH100 values in association with positive IgG-ACA and one patient had positive lupus anticoagulant.
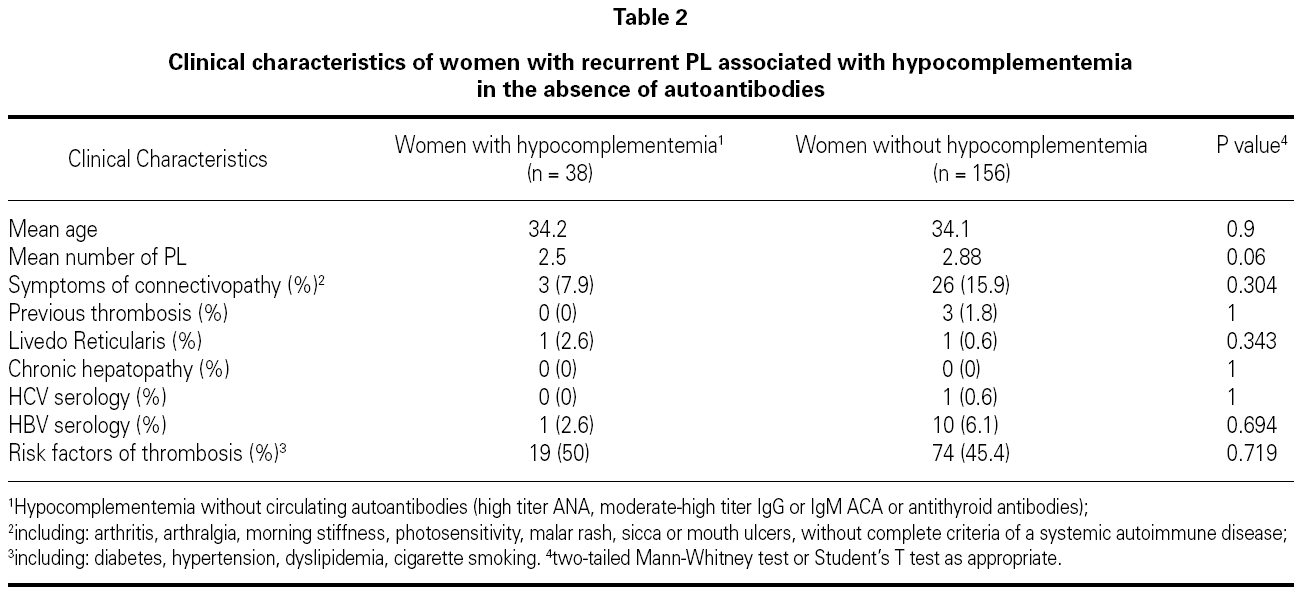
Overall, 38 (18.9 %) patients with recurrent PL had data of hypocomplementemia in the absence of autoantibodies in perypheral blood in a significantly higher frequency than parous healthy women (n = 2, p = 0.049). Clinical characteristics of patients with hypocomplementemia in the absence of autoantibodies are shown in table 2.
When we compared the levels of complement factors of women with recurrent PL without autoantibodies to women with recurrent PL and positive autoantibodies, there were not significant differences. When the data for complement levels were analyzed by gestational age at pregnancy loss there were not significant differences between women with early PL (< 10 weeks) and late PL (> 10 weeks).
DISCUSSION
Even though pregnancy presents an immunological riddle in that the mother does not reject semi-allogenic fetus, few alterations of this critical balance appear to occur during normal pregnancy, as most pregnancies proceed to term without major complications. Disturbances in this delicate balance may, however, play an important role in serious pregnancy-related disorders, most prominently in cases with recurrent PL1.
A fully active complement system deriving from the maternal circulation as well as from local production by various cell sources is present in the placenta8. The role of this system at the placental level, as in any other tissue in the body, is to protect both the fetus and the mother against infectious and other toxic agents. As fetal tissues are semi-allogenic and alloantibodies commonly develop in the mother, the placenta is potentially subject to complement-mediated immune attack at the feto-maternal interface with the potential risk of PL. Experimental observations suggest that inappropriate complement activation during pregnancy impairs this type of immunoregulation8-11.
A role for complement as effector of PL has been suggested in different clinical settings. Within APS associated PL, involvement of the complement system has been demonstrated. Experiments performed in a mouse model of APS showed that activation of the complement cascade is required to induce PL and thrombosis12-13. Interestingly enough, heparin, the standard anticoagulant treatment used to prevent obstetric complications in patients with APS14 has been shown to have "anti-complementary effects"15-19. Even in the absence of detectable anticoagulation, heparin prevented antiphospholipid antibody-induced PL and inhibited systemic complement activation20.
Although formation of pathogenic antibodies with activation of the classical pathway may have a role, this mechanism fails to characterize the majority of cases with unexplained recurrent PL. It is, however, possible that complement activation is also involved in other causes of recurrent PL. Indeed, in antibody-independent mouse models, it has been recently demonstrated that complement activation is a required event in the pathogenesis of placental and fetal injury2,21. A few studies have associated early and late PL with the onset of hypocomplementemia and, in some conditions, with complement deposition in the placenta22-23.
With the limitations of a retrospective study using chart review, we detected a group of women with recurrent PL and significant hypocomplementemia, including low C3, low C4, low FB or low CH100 (with normal values of C3, C4 and FB), without presence of autoantibodies or other secondary conditions that could explain low concentrations of complement factors. Low CH100 with normal values of C3, C4 and FB might be indicative of deficiency of other complement factors such as C5 (not tested in our study). Asymptomatic partial primary complement deficiencies should be taken into account as a possible explanation for the low levels of complement factors observed in some of these patients. However, that a subgroup of women with unexplained recurrent PL had hypocomplementemia, in the absence of known causes of complement consumption, might also suggest activation of maternal complement factors.
Whether hypocomplementemia is the cause or effect of PL in these women can't be explained by this study. In this regard, further prospective studies in a larger number of patients are encouraged to establish whether the complement system might be implicated in mechanisms of recurrent PL in women with unexplained recurrent PL independently of the presence of detectable serum autoantibodies.
ACKNOWLEDGEMENTS
The authors acknowledge Margarita Rodriguez-Mahou for the performance of autoantibody detection tests at the Autoimmunity Section. JC was supported by Fundacion Salud 2000 (Ayudas Serono 2005), Madrid, Spain.
Correspondence:
Dr. J. Carbone
Department of Immunology
University Hospital Gregorio Marañon
Dr Esquerdo 46
28007 Madrid. Spain
Phone: +34 91 4265180
FAX: +34 91 5866698
E-mail: carbone@teleline.es