Amaranthus retroflexus (Redroot Pigweed) is one of the main sources of allergenic pollens in temperate areas. Polcalcin is a well-known panallergen involved in cross-reactivity between different plants. The aim of this study was the molecular cloning and expression of polcalcin, as well as evaluating its IgE-reactivity with A. retroflexus sensitive patients’ sera.
MethodsAllergenic extract was prepared from A. retroflexus pollen and the IgE-reactivity profile was determined by ELISA and immunoblotting using sera from twenty A. retroflexus sensitive patients. Polcalcin-coding sequence was amplified by conventional PCR method and the product was inserted into pET-21b(+) vector. The recombinant protein was expressed in E. coli BL21 and purified by metal affinity chromatography. The IgE-binding capability of the recombinant protein was analyzed by ELISA and immunoblotting assays, and compared with crude extract.
ResultsOf 20 skin prick test positive patients, 17 patients were positive in IgE-specific ELISA. Western blotting confirmed that approximately 53% of ELISA positive patients reacted with 10kDa protein in crude extract. The A. retroflexus polcalcin gene, encoding to 80 amino acid residues was cloned and expressed as a soluble protein and designated as Ama r 3. The recombinant polcalcin showed rather identical IgE-reactivity in ELISA and western blotting with 10kDa protein in crude extract. These results were confirmed by inhibition methods, too.
ConclusionThe recombinant form of A. retroflexus polcalcin (Ama r 3) could be easily produced in E. coli in a soluble form and shows rather similar IgE-reactivity with its natural counterpart.
Pollens from the Amaranthaceae family have been reported as a major source of pollinosis in the United States, Europe, and Asia. Among this genus, pollens of Amaranthus retroflexus (Redroot Pigweed), a common species of this family, is regarded as a major source of respiratory allergy in semi-desert countries.1–5 Previous findings showed that A. retroflexus is a causative agent of allergic asthma and rhinitis in Iran and up to 69% of patients are sensitive to pollens of this weed.6 Protein analysis of A. retroflexus pollen revealed several allergenic components ranging from 10 to 85kDa and sera from allergic patients mainly show IgE-reactivity with 10, 15, 18, 39, 45, and 85kDa proteins of this pollen. Since the abundantly expressed low molecular allergens may play a major role in the development of respiratory allergy symptoms, therefore, the initial effort is characterization of these types of allergens. In this regard, Ama r 1 and Ama r 2 were previously introduced as allergens of this weed.7–10 Profilin (Ama r 2) with molecular weight of 15kDa was the first panallergen from this source which was reported in 2011.10 Ama r 1 is a member of Ole e 1-like protein family of proteins with molecular weight of 18kDa which has recently been described, and its IgE-reactivity was confirmed in more than 38% of A. retroflexus allergic patients.9 Overall, in spite of a high rate of sensitization to pollens from Amaranthus species in different areas of the world, up to now, limited studies have been conducted on determination of the molecular properties of A. retroflexus allergens. In the current study, we evaluated the IgE-immunoreactivity of the crude extract of A. retroflexus pollen with sensitive patients’ sera and following the molecular cloning and expression of polcalcin as a possible allergen, its IgE-reactivity was assessed.
Materials and methodsCrude extract preparationDefatted pure pollen of A. retroflexus was purchased from Greer Laboratories, USA. For protein extraction, 0.5g of the pollen was mixed with 5ml of phosphate buffered saline (PBS, 0.15M, pH 7.4) and agitated overnight on an orbital shaker, at 4°C. The supernatant was separated by centrifugation at 13000×g for 20min and filtered through a 0.22μm PVDF membrane. Then the clear supernatant was dialyzed against 20mM potassium phosphate buffer pH 8.0, overnight at 4°C. The protein concentration of the crude extract was measured by Bradford's method.11
Patients’ sera and skin prick test (SPT)Twenty patients from Firouzabadi general hospital (Tehran, Iran) who mentioned a history of rhinitis or rhinoconjunctivitis during summer were enrolled in this study. The patients were requested to complete a detailed questionnaire as well as an informed consent. They were considered allergic to A. retroflexus if they reported a history of at least one ophthalmic, nasal, or respiratory symptom to common aeroallergens and demonstrated a typical positive skin prick test (SPT) with A. retroflexus commercial extract (Greer Laboratory, USA). Moreover, ten healthy volunteers who showed no significant signs of reactivity to A. retroflexus pollen extract in SPT, were involved as negative controls. Serum samples were collected from all participants and stored at -20°C. The human ethics committee of Shahrkord University of Medical Sciences approved the study protocol (ethics code # IR.SKUMS.REC.1394.155).
Specific enzyme-linked immunosorbent assays (ELISA) with crude extractTo evaluate the specific IgE-reactivity of the patients to the crude extract, patients’ sera were assessed by an in-house developed indirect ELISA as previously described in duplicate manner.7 In brief, 2μg (100μl) of the crude extract was coated in ELISA wells (Nunc MaxiSorp™, Denmark) using bicarbonate buffer pH 9.6 (15mM Na2CO3 and 35mM NaHCO3) and the plates were incubated overnight at 4°C. After washing with PBS containing 0.05% Tween 20 (PBS-T), the plates were blocked with 250μl of 2% bovine serum albumin (BSA) in PBS (3h, 37°C). Afterwards 100μl of 1:5 diluted patients’ sera was added and the plates were incubated for another 3h at 37°C. After an extensive wash, 100μl of 1:1000 diluted biotinylated anti-human IgE antibody (Abcam, USA) in 1% BSA was added to each well and the plates were incubated for 2h at 37°C. Following an extensive washing, 100μl of 1:2000 diluted horseradish peroxidase (HRP)-conjugated streptavidin (Sigma–Aldrich, USA) was added to each well and the plates were incubated for 45min at 37°C. Following five washes with PBS-T, 100μl of chromogenic TMB/H2O2 substrate was added to each well and the plates were incubated for 15min in the dark. Color development was stopped by the addition of 100μl of 2N H2SO4. Finally, optical density of the wells was measured at 450nm versus 630nm as reference filter with an ELISA reader.
Cloning and expression of polcalcinTotal RNA was isolated from 0.1g of the de-fatted pollen using TRIzol® reagent (Invitrogen) according to the Chomczynski and Sacchi method.12 Then the first strand cDNA was synthesized using a commercial cDNA synthesis kit (Parstous, Iran) according to the manufacturer's instructions and applied as template in RT-PCR. The primers used for amplification of cDNA were designed according to previously determined sequences of polcalcin from Chenopodium album (Che a3) as well as other members of the Chenopodiacea or Amaranteacea family of weeds.13 The sense primer (5′-ATAGGATCCAGCTGCTGAGGATACACCTCA-3′) contained a BamHI restriction site and the reverse primer (5′-ATACTCGAGGAAGATCTTGGAAACATCTTA-3′) contained an XhoI restriction site, as underlined. The protein coding sequence was amplified with conventional PCR. In brief, the contents were denatured at 95°C for 5min, and the desired sequence was amplified using 30 cycles of 94°C for 30s, 55°C for 45s, and 72°C for 45s and a final elongation at 72°C for 10min. The PCR products were electrophoresed on a 2% agarose gel and the PCR amplicon was cleaned up using a commercial kit (YTA Miniprep Kit, Iran). The PCR products as well as the bacterial expression vector (pET-21b, from Invitrogen, USA) were digested with XhoI and BamHI restriction enzymes, according to the manufacturer's protocol (Fermentas, Lithuania). Purified fragments were ligated with T4 DNA ligase and the product was transformed to E. coli BL21 (DE3) (Novagen, USA) competent cells. Finally, ampicillin-resistant colonies were selected for plasmid extraction and DNA sequencing.
Expression and purification of the recombinant polcalcinThe purified pET-21-Polcalcin construct was transformed into E. coli BL21 cells. Briefly, a fresh colony was inoculated into 2ml of Luria-Bertani (LB) medium containing 100μg/ml of ampicillin and incubated at 37°C. When the OD600nm reached 0.4, the expression of the recombinant protein was induced by the addition of isopropyl β-d-thiogalactopyranoside (IPTG) to a final concentration of 1mM, and the bacterial cells were incubated at 18°C with constant shaking at 250 RPM on an orbital shaker, overnight. Consequently, the cells were harvested by centrifugation at 3000×g for 10min at 4°C. The bacterial cells were resuspended in lysis buffer (50mM Tris–HCl PH 8.0 supplemented with 5mM 2ME and 100mM NaCl), and then subjected to five freeze-thaw cycles. The lysate was centrifuged at 3000×g for 10min at 4°C and the recombinant protein was purified by nickel affinity chromatography with a 6His Trap column (Ni-IDA Resin, Parstous, Iran) according to the manufacturer's instructions. The purity and concentration of the purified fractions were evaluated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and Bradford assay.
IgE-specific ELISA for the recombinant polcalcinThe specific IgE-reactivity of the patients’ sera with the purified recombinant protein was measured by indirect ELISA as described above, except that the ELISA wells were coated with 0.5μg per well of the purified protein.
IgE-immunoblotting and inhibition assaysThe contents of crude extract and purified recombinant protein were studied by electrophoresis in 15% polyacrylamide gel under reducing conditions as described by Laemmli et al.14 The resolved proteins were electro-transferred to polyvinylidene difluoride (PVDF) membranes (Millipore, Bedford, USA) at 0.8mA/cm2 within 50min using a semi-dry transfer apparatus (PeQlab Biotechnologie GmbH, Germany). In brief, after protein transfer, the membranes were cut to lanes and blocked with 2% BSA within 3h on a rocker at 37°C. Each lane (either containing crude extract or recombinant protein) was incubated with 1:5 dilutions of the serum samples overnight on a rocker at 4°C. After washing with PBS-T (three times, each for 5min), biotinylated anti-human IgE (1:1000 in 1% BSA) was added and the lanes were incubated for 2h at 37°C. Unbound antibodies were removed by washing with PBS-T, afterwards, the lanes were incubated with 1:2000 dilution of HRP-linked streptavidin (Sigma–Aldrich, USA) for 1h at 37°C. After several extensive washings with PBS-T, the lanes were incubated with electro chemiluminescent substrate (Parstous, Iran) for 2min, and the chemiluminescent bands were then visualized by Chemidocumentation System (Labtech, FUSION FX, England).
To study the cross-inhibition among A. retroflexus natural and recombinant polcalcin, 100μl of patients pooled sera was added to similar volumes of the crude extract (35μg/ml) or recombinant polcalcin (10μg/ml), or 0.1% BSA (as control) and incubated for 2h at 4°C on a rocker. Then the IgE-reactivity of the pre-incubated sera with pollen crude extract proteins were assessed by western blotting method.
Molecular modeling of polcalcin structureFollowing the molecular cloning of polcalcin, its amino acid sequence was submitted at the SWISS-MODEL server homology modeling pipeline (https://swissmodel.expasy.org/) to generate its ribbon structure, then the quality of the constructed model was evaluated by QMEAN SERVER (http://swissmodel.expasy.org/qmean/cgi/index.cgi). Possible regions of IgE binding epitopes were predicted by IEDB serve (https://www.iedb.org/) and displayed on the constructed model.
Statistical analysisThe optimal cut-off values for each ELISA method, with 95% confidence intervals (CI) were determined by the receiver operating characteristic (ROC) curve analysis. The immunoreactivity of antigens was defined as frequency percentage of the positive and negative results. Statistical analysis and graphs were prepared using Graph Pad Prism version 6.0 (GraphPad Software, La Jolla, CA, USA).
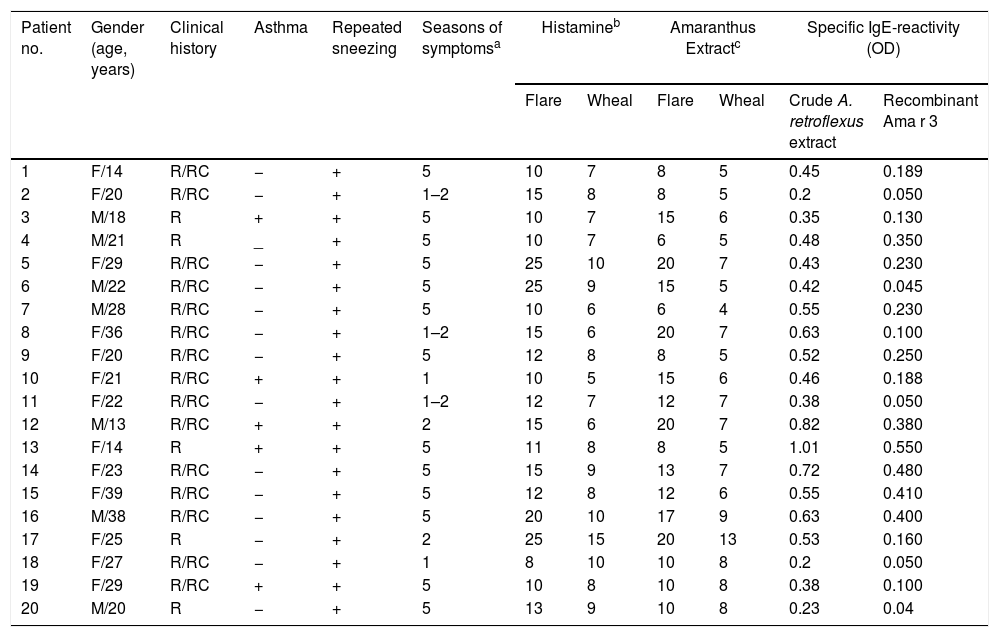
ResultsPatients and skin prick testsThe study was performed on 20 A. retroflexus allergic patients (13 females and 7 males) with median age of 22.5 years (ranging from 13 to 39 years) and 10 healthy individuals. All patients complained of rhinitis or rhinoconjunctivitis and showed positive SPT to the A. retroflexus pollen extract with a mean wheal diameter of 6.5±2.00mm. Demographic data and clinical findings of the patients are summarized in Table 1.
Clinical characteristics, IgE-reactivity and SPT responses of the A. retroflexus allergic patients.
| Patient no. | Gender (age, years) | Clinical history | Asthma | Repeated sneezing | Seasons of symptomsa | Histamineb | Amaranthus Extractc | Specific IgE-reactivity (OD) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Flare | Wheal | Flare | Wheal | Crude A. retroflexus extract | Recombinant Ama r 3 | ||||||
| 1 | F/14 | R/RC | − | + | 5 | 10 | 7 | 8 | 5 | 0.45 | 0.189 |
| 2 | F/20 | R/RC | − | + | 1–2 | 15 | 8 | 8 | 5 | 0.2 | 0.050 |
| 3 | M/18 | R | + | + | 5 | 10 | 7 | 15 | 6 | 0.35 | 0.130 |
| 4 | M/21 | R | _ | + | 5 | 10 | 7 | 6 | 5 | 0.48 | 0.350 |
| 5 | F/29 | R/RC | − | + | 5 | 25 | 10 | 20 | 7 | 0.43 | 0.230 |
| 6 | M/22 | R/RC | − | + | 5 | 25 | 9 | 15 | 5 | 0.42 | 0.045 |
| 7 | M/28 | R/RC | − | + | 5 | 10 | 6 | 6 | 4 | 0.55 | 0.230 |
| 8 | F/36 | R/RC | − | + | 1–2 | 15 | 6 | 20 | 7 | 0.63 | 0.100 |
| 9 | F/20 | R/RC | − | + | 5 | 12 | 8 | 8 | 5 | 0.52 | 0.250 |
| 10 | F/21 | R/RC | + | + | 1 | 10 | 5 | 15 | 6 | 0.46 | 0.188 |
| 11 | F/22 | R/RC | − | + | 1–2 | 12 | 7 | 12 | 7 | 0.38 | 0.050 |
| 12 | M/13 | R/RC | + | + | 2 | 15 | 6 | 20 | 7 | 0.82 | 0.380 |
| 13 | F/14 | R | + | + | 5 | 11 | 8 | 8 | 5 | 1.01 | 0.550 |
| 14 | F/23 | R/RC | − | + | 5 | 15 | 9 | 13 | 7 | 0.72 | 0.480 |
| 15 | F/39 | R/RC | − | + | 5 | 12 | 8 | 12 | 6 | 0.55 | 0.410 |
| 16 | M/38 | R/RC | − | + | 5 | 20 | 10 | 17 | 9 | 0.63 | 0.400 |
| 17 | F/25 | R | − | + | 2 | 25 | 15 | 20 | 13 | 0.53 | 0.160 |
| 18 | F/27 | R/RC | − | + | 1 | 8 | 10 | 10 | 8 | 0.2 | 0.050 |
| 19 | F/29 | R/RC | + | + | 5 | 10 | 8 | 10 | 8 | 0.38 | 0.100 |
| 20 | M/20 | R | − | + | 5 | 13 | 9 | 10 | 8 | 0.23 | 0.04 |
The polcalcin coding sequence of A. retroflexus was successfully subcloned in the multiple cloning region of the bacterial plasmid using directional PCR cloning method and approved by further confirmation assays. Analysis of the insert showed an open reading frame with 256-bp nucleotides encoding to 80 amino acid residues with a predicted molecular mass of approximately 12kDa, which was designated as Ama r 3.
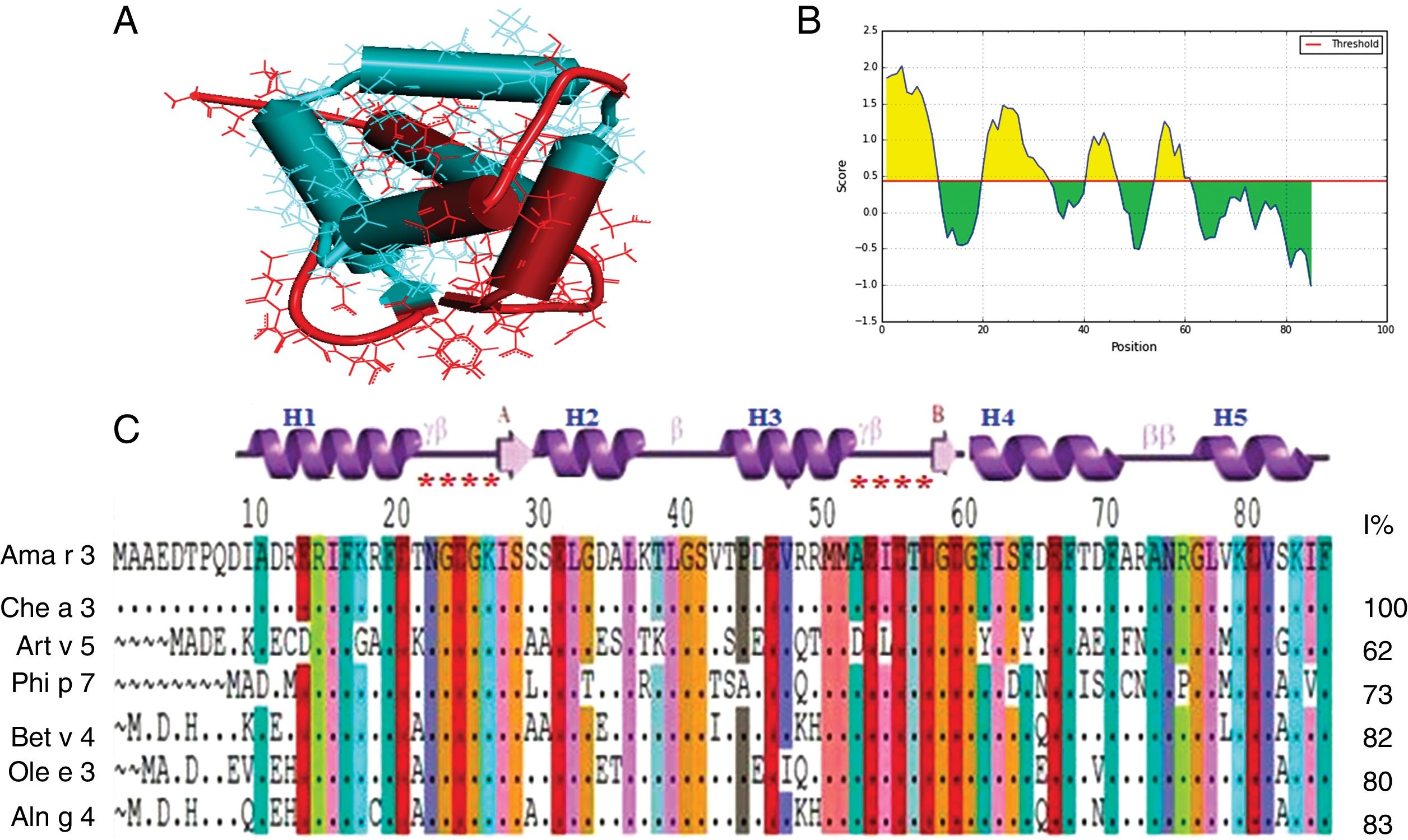
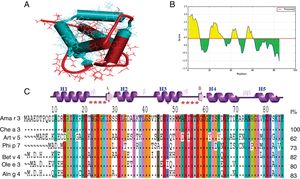
The QMAN score for PDB structure obtained from SWISS-MODEL server was in reliable range (−1.61) and Z-score for predicted model was within the range of reported scores for native proteins with similar size. At least four B-cell epitopes were predictable on Ama r 3 (Fig. 1). Comparison of the deduced amino acid sequence of the cloned protein with polcalcin of other allergenic plants, revealed a high sequence identity (95%) with Che a3 (Fig. 1C). The recombinant polcalcin was expressed in E. coli BL21 (DE3) in soluble form in the supernatant of the bacterial lysate as a fusion protein with 6His-tagged C-terminus. It was further purified by Ni2+ affinity chromatography yielding approximately 1.5mg of recombinant protein per liter of the bacterial expression medium. The apparent molecular weight of the fusion protein in SDS-PAGE was approximately 12kDa (Fig. 2A).
Representation of B-cell epitopes on constructed structure of polcalcin. (A) Ribbon structure of polcalcin was generated by SWISS-MODEL server and predicted B-cell epitopes were represented by blue color. (B) Bepipred linear epitope prediction of polcalcin, the highest peak region shows most potent B cell epitopes, in which the X-axis and Y-axis indicate the position and score, respectively. (C) Comparison of the amino acid sequence of A. retroflexus polcalcin (Ama r 3) with allergic polcalcins from other plants. The percentages of the identity of amino acid sequence of Ama r 3 with other members of the polcalcin family are shown at the end of each allergen amino acid sequence. Chenopodium album (Che a 3, Q84V36); Artemisia vulgaris (Art v 5, A0PJ17); Phleum pratens (Phi p 7, Y17835); Betula verrucosa (Betv4, Y12560. X87153); Olea europaea (Ole e3), and Alnus glutinosa (AIng 4, Y17713).
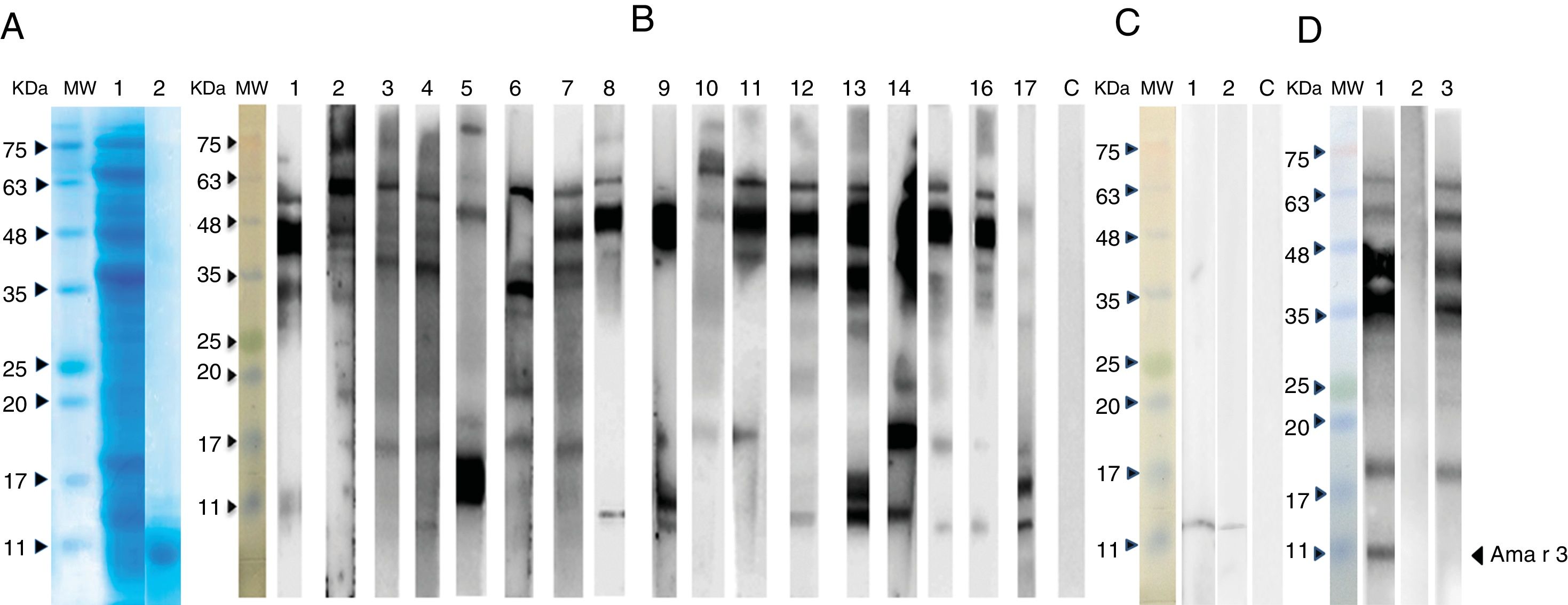
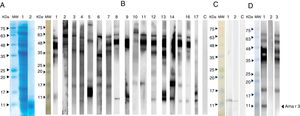
SDS-PAGE and immunoblotting of A. retroflexus pollen extract with patients’ sera. (A) Coomassie Brilliant Blue-stained SDS-PAGE of A. retroflexus crude extract and purified recombinant Ama r 3 on 15% gel. (B) Lanes 1–17 demonstrate IgE-immunoblotting of crude extract with allergic patients’ sera. (C) IgE-immunoblotting of recombinant Ama r 3. In lanes 1–6, rAma r 3 was probed with individual allergic patients’ sera; lane C, negative control. MW stands to molecular weight marker (Sinnagen, Iran) and lane C refers to probing with pooled sera with negative controls. (D) Western blot inhibition assay of A. retroflexus. Lane 1: probing of A. retroflexus crude extract strip with patients’ pooled sera; Lane 2: Probing of A. retroflexus crude extract strip with crude extract-pre-absorbed sera. Approximately 35μg of A. retroflexus crude extract was added to 100μl of the patients’ pooled sera and incubated on a shaker for 2h and used for blotting; Lane 3: Probing of A. retroflexus crude extract strip with recombinant Ama r 3-pre-absorbed sera. Approximately 10μg of purified rAma r 3 was added to 100μl of the patients’ pooled sera and incubated 2h on a shaker and used for blotting. MW: molecular weight marker (Sinnagen, Iran).
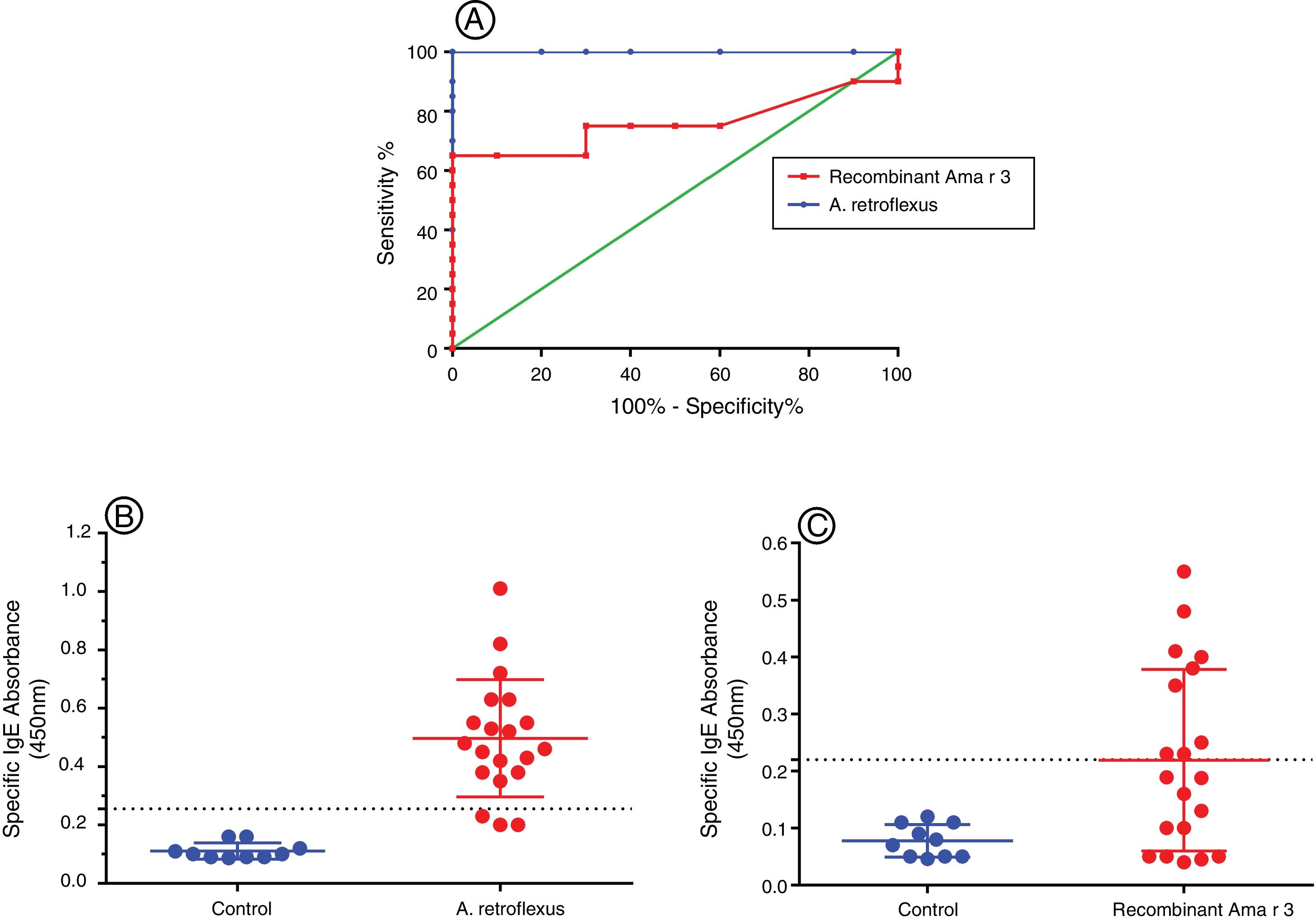
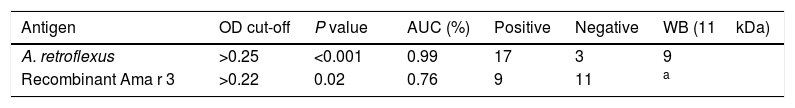
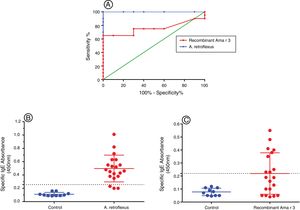
In this study, we evaluated specific IgE-reactivity of the pollen proteins and recombinant antigens by indirect ELISA. The cut-off values were determined using ROC curve analysis (Fig. 3A). AUC for crude extract proteins and recombinant antigens were 0.99% (P<0.001) and 76% (P=0.02), respectively (Table 2 and Fig. 3A). The positive frequencies for crude and recombinant antigens were 17 (85%) and nine (45%), respectively (Table 2, Fig. 3B and C).
(A) Receiver operator characteristic (ROC) curve for A. retroflexus and recombinant Ama r 3 antigens. The values for the area under the ROC curve were 0.99 for the A. retroflexus and 0.76 for recombinant Ama r 3 in ELISA. (B) Dot plots showing the absorbance values (450nm) of ELISA results for discrimination of the IgE-reactivity of (a) A. retroflexus crude extract and (b) recombinant Ama r 3 of A. retroflexus sensitive patients versus healthy controls.
Comparison of specific IgE-reactivity of A. retroflexus and recombinant Ama r 3 with different cut-off points of absorbance (450nm) using ELISA test.
| Antigen | OD cut-off | P value | AUC (%) | Positive | Negative | WB (11kDa) |
|---|---|---|---|---|---|---|
| A. retroflexus | >0.25 | <0.001 | 0.99 | 17 | 3 | 9 |
| Recombinant Ama r 3 | >0.22 | 0.02 | 0.76 | 9 | 11 | a |
WB: immunoreactivity in western blotting with 11kDa protein in crude extract.
SDS-PAGE of the crude extract demonstrated several protein bands ranging from 10 to 85kDa, accompanied with a faint smear of proteins. Accordingly, several IgE-reactive proteins were observed in western blotting in which the most prominent bands belonged to the molecules located at 40–60kDa region. Several IgE-reactive bands with different molecular weights were also observed in other regions ranging from apparently 10 to 85kDa. Interestingly, nine out of 17 (53%) of the patients reacted with an approximately 10kDa protein in the crude extract (Fig. 2B). Furthermore, the pooled sera of the patients exhibited considerable IgE-reactivity with the recombinant protein in western blotting, while, pooled sera from controls did not show significant reaction.
IgE-immunoblotting and immunoblot inhibition of rAma r 3All of the serum samples were also tested for IgE-reactivity with the recombinant Ama r 3 by ELISA. We also determined the IgE-reactivity of recombinant polcalcin with western blotting. The results were consistent with the immunoreactivity of the 10kDa band observed in A. retroflexus crude extract.
Immunoblot inhibition assays showed that pre-incubation of serum samples with rAma r 3 almost completely inhibited the IgE-binding to a protein band with an apparent molecular weight of 10kDa. Altogether, in vitro inhibition assays revealed a similar IgE-reactivity for rAma r 3 and its natural counterpart in A. retroflexus crude extract. In addition, further inhibition assays indicated that pre-incubation of serum samples with the native crude extract of A. retroflexus pollen completely inhibited the IgE-binding to the native immunoreactive protein; while, pre-incubation of the pooled serum with inert proteins such as BSA did not affect IgE-reactivity of the sera with total extract and rAma r 3 (Fig. 2D).
DiscussionPigweed is a well-known aeroallergen in areas with temperate, dry or semi-desert climate such as Saudi Arabia, Kuwait, India, and Iran.1,5,15 In this study, we focused on the characterization, cloning and production of a new allergen from A. retroflexus pollen. This allergen, which is a member of polcalcin family of proteins (Ca2+-binding proteins), was designated as Ama r 3.
The open reading frame of Ama r 3 encoded to an approximately 12kDa protein, which correlated with the molecular weight of previously characterized plant polcalcins. Amino acid sequence analysis revealed that Ama r 3 has a high degree of identity with polcalcin from the most common allergenic regional plants in Iran. In particular, it showed 95% identity with Che a 3.
The IgE-reactivity of the patients’ sera with A. retroflexus proteins were assessed by ELISA and western blotting. Most of the samples showed significant reactivity with crude extract proteins in both experiments. The reactivities in ELISA were consistent with those obtained in the IgE-immunoblotting assays.
Ca2+-binding proteins contain a variable number of EF-hand motifs which consist of two vertically placed alpha helices and an inter-helical loop forming a single calcium-binding site. Most of the Ca2+-binding allergens described so far represent low-molecular-weight, water-soluble proteins mostly ranging from 8 to 25kDa.16 Some of these allergens such as Bet v 3,17 Bet v 4,18,19 Aln g 4,20 and Ole e 321 are found in pollens of trees, while, Cyn d 7,22,23 and Phi p 724 are well-known allergens in grasses, and Bra n 1, Bra n 2, Bra r 1, Bra r 225 and Che a 313 are found in weeds.
The immunoblotting of A. retroflexus pollen extract with sera from the patients also indicated an IgE-binding protein band with an estimated MW of 10kDa. The MW of polcalcin from various plant sources differ in size, so that polcacin of Olea europaea pollen (Ole e 3) is 9.2kDa, while similar allergens from C. album pollen (Che a 3) is 9.5kDa and from Phleum pratense pollen (Phl p 7) is 8.6kDa.13,21,24 Obviously, these variations in the mass of similar proteins refers to diversities of the amino acid residues, as well as glycosylation and other post translational modifications of the mentioned allergens. Immunoblotting of A. retroflexus pollen extract with patients’ sera, also revealed an approximately 10kDa IgE-binding protein which was not studied before (Fig. 2B). Based on previous studies on similar plants, we considered that it could be polcalcin. This probability was confirmed by cloning of the protein and different immunoassays. Polcalcin was cloned and the IgE-binding capability of the purified recombinant protein with sera from A. retroflexus allergic patients was evaluated by specific ELISA and immunoblotting assays in order to confirm that they have similar characteristics. Achievement of similar results for native and recombinant protein, in parallel to finding in the inhibition assays confirmed that the produced recombinant protein was correctly folded and could bind to patients’ specific IgE, similar to the natural counterpart in A. retroflexus extract. The results of immunoblotting assays for natural polcalcin were in line with those obtained from recombinant Ama r 3. A nearly complete inhibition of IgE-binding to natural A. retroflexus polcalcin was also obtained after pre-incubation of pooled serum with purified r Ama r 3. Taken together, it seems that r Ama r 3 is comprised of a protein structure with IgE-epitopes similar to those of its natural counterpart.
Recently, cross-reactivity of the A. retroflexus pollen with other allergenic plants has been described. Pollen allergens with two EF-hand structures from trees (Aln g 4, Bet v 4, Ole e 3), grasses (Cyn d 7, Phi p 7) and weeds (Bra n 1, Bra n 2. Bra r 1, Bra r 2, Che a 3) are categorized among the most cross-reactive ones. Interestingly, up to now no significant cross-reactivity was found among the two EF-hand, three EF-hand (e.g. Bet v 3) and four EF-hand (Jun o 2) pollen allergens.16
The expression of recombinant proteins in bacterial hosts sometimes results in an inclusion body which requires denaturation and subsequent refolding in order to obtain the stable conformation, which is a requisite for the IgE-binding reactivity of recombinant allergens. In this study, Ama r 3 was successfully expressed in E. coli as a soluble molecule. During the process of expression and after induction with IPTG, the temperature of the medium was lowered to 18°C to obtain the soluble form of the protein. Surely, the expression of recombinant allergens in soluble form could facilitate providing enough amount of them, in an appropriate conformation, suitable for further in vitro and in vivo studies.
Conflict of interestThe authors have no conflict of interest to declare.
AcknowledgmentThis study was supported by grant number 26136 from Iran University of Medical Sciences.