INTRODUCTION
The Down syndrome (T21) is a chromosome pathology characterized for a set of alterations still not well defined. Children from mothers with an age below 25 years have 1:2,000 risk of having the problem, increasing with the maternal age until an incidence of 1:50 at the 40 years. The children with Down syndrome present characteristic morphologic alterations. About 1/3 have congenital cardiac anomalies. A higher incidence of endocrine alterations, particularly of the thyroid gland, exists as it is verified an increase trend for deafness and atlanto-occipital instability. The children with Down syndrome equally present increased incidence of malignant diseases of the hematopoetic system (1).
It is referred by diverse authors an increased risk for infections and a comparatively higher morality to the general population (1). Among other anomalies, it has been shown altered serum concentrations of some of the subclasses of IgG, namely IgG2 and IgG4 (2), with inferior values to the ones of the general population. The published studies in general proceed to the matching between samples from children with T21 and the normal control pupulation (2, 3). Studies with groups of children, with and without T21, with the same parenteral origin are not referred in the literature.
The importance of the determination of IgG serum concentrations in clinical practice is being a matter of discussion. Evidences point out that they are useful in certain particular situations (3, 4), while other studies do not advice their routine performance (5).
MATERIAL AND METHODS
In our study were included 206 caucasian children, divided in three different groups. One group included children with T21, another formed by their siblings and a third with otherwise considered normal children. In the first group were included children with T21 followed in the Development Consultation of the University Clinic of Pediatrics. These childrens are accomplishing for a follow-up protocol with incidence in all aspects of their medical and daily life, being well integrated regarding familiar, scholar and social aspects. The T21 group was constituted by 79 individual, 39 girls and 40 boys, with an average age (SD) of 4.6 (3.0) years and a variation between 1 and 14 years. The group of the siblings (FT21) included 73 individuals, 36 girls and 37 boys, with an average age (SD) of 7.5 (4.8) years, variation between 1 and 18 years. Fifty-four normal children formed a third group, 31 girls and 23 boys with an average age (SD) of 3.9 (3.5) years, variation between 1 and 12 years. Exclusion criteria involved all children suffering from acute or chronic systemic or respiratory infectious diseases. Samples from normal children were collect from those undergoing minor surgery procedures or followed in the outpatient clinic after hospital discharge. Informed consent was obtained in all cases.
The blood samples were drawn by venopuncture according to standard procedures, centrifuged and the serum stored at 20 °C until submitted to laboratorial analysis. Serum samples processing took place in the Pediatrics Laboratory of the University School of Medicine of Hospital Santa Maria.
The IgG concentration was determined by nephelometry (Behring Nephelometer 100 Analyzer, Messer Greisheim GmbH, Frankfurt, Germany), and IgG subclasses levels by radial immunodifusion using the Radial Immunodifusion Kit BINDARID 3 plate kits (The Binding Site Limited) according to protocol procedure defined by the manufacturer. Those values under two standard deviation below of the mean for normal children were considered as low serum concentrations.
Three age sub-groups in the groups of SD and FTSD were considered, in function of the homogeneity of the variation of serum concentrations in each one of them: below four years old, between four and eight years and above eight years old. The normality of the distribution of the values of IgG subclasses serum concentrations was verified in each group with the test of Kolmogorov-Smirnov. If normal distribution was verified then comparative matching between the diverse age sub-groups using the test t-Student was made. In alternative, the Mann-Withney test was applied.
The results were expressed graphically using clustered boxplot showing median and quarter centile values after logarithmic transformation of data. Statistical calculations and graphic analysis were made with SPSS version 10.
RESULTS
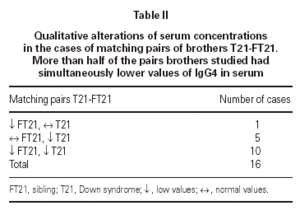
Only in two cases, both in the group of control, lower serum concentrations of total IgG (under 2 SD) were verified, both with reduction of the IgG4. In the group of T21 we did not identified any cases of total IgG deficiency, despite the fact some individual showed lower values in several subclasses (IgG2 and IgG4), mostly due to compensatory higher values of IgG1 and IgG3 subclasses. We compared the number of the individual IgG subclass deficiency for each group (table I). Only IgG2 and IgG4 demonstrated a relevant number of deficiency cases. None of the pairs of brothers T21-FT21 showed concordance in the variation of IgG2 serum concentration towards deficiency. Among T21 group, 32/77 children evidenced low values of IgG4, and in 16 pairs of brothers T21-FT21 that we analyzed, 10/16 had low serum concentrations of IgG4 in both brothers (table II).
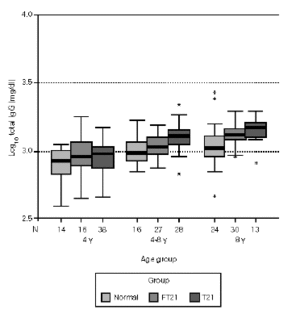
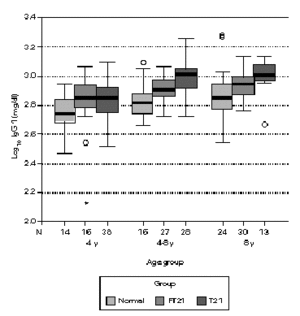
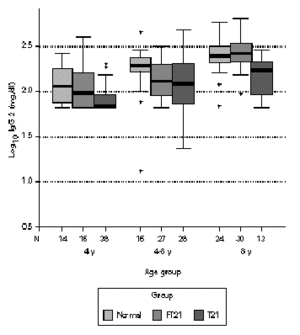
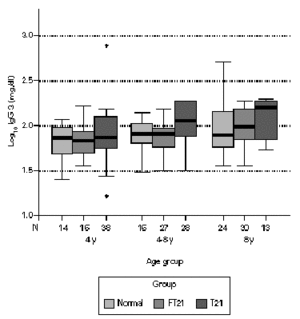
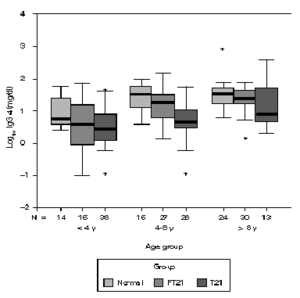
Considering the different age sub-groups, statistical significant differences between the three groups were found. Down syndrome children showed tendency to present higher values of total IgG, with statistical significance above four years old in relation to the other two groups (P < 0.05). In the oldest group of siblings, there also a significant difference to the normal population (P < 0.01). In relation to IgG1, Down syndrome children and their siblings show statistically higher values to the normal population in all age groups. Above four years old, T21 group has a significant different to the rest (P < 0.01). Down syndrome children present statistically lower values of IgG2 to the normal population below eight years old (P < 0.05) and to their siblings in the oldest groups (P < 0.01). Between 4-8 years, FT21 group was identified has having also lower serum concentration, comparing to the normal population. Down syndrome children present statistically higher values of IgG3 to the normal population above four years old and to their siblings groups between 4 and 8 years old (P < 0.01); the siblings group has higher values than normal population in the oldest group (P < 0.05). In relation to IgG4, there is no difference between normal and siblings group. Down syndrome group revealed statistically lower values, compared with normal population below 8 years old (P < 0.01), and their siblings in the group between 4 and 8 years old (P < 0.05) (figs. 1-5).
Figure 1.--Comparison of total serum IgG levels (mg/dl) according to age. Data in clustered boxplot show the median values for each case. Down syndrome children show higher values, with statistical significance above 4 years old for the normal and sibling groups. In the oldest group of siblings, there is also a significant difference to the normal population (p < 0.05).
Figure 2.--Comparison of total serum IgG1 levels (mg/dl) in three different groups, according to age; data in clustered boxplot show the median values for each case. Down syndrome children and their siblings show statistically higher values to the normal population (p < 0.05).
Figure 3.--Comparison of total serum IgG2 levels (mg/dl) in three different groups, according to age; data in clustered boxplot show the median values for each case. Down syndrome children present statistically lower values to the normal population below 8 years old and to their siblings in the oldest groups (p < 0.05).
Figure 4.--Comparison of total serum IgG3 levels (mg/dl) in three different groups, according to age; data in clustered boxplot show the median values for each case. Down syndrome children present statistically higher values to the normal population above 4 years old and to their siblings groups between 4 and 8 years old; the siblings group has higher values than normal population in the oldest group (p < 0.05).
Figure 5.--Comparison of total serum IgG4 levels (mg/dl) in three different groups, according to age; data in clustered boxplot show the median values for each case. There is no difference between normal and siblings groups. Down syndrome children present statistically lower values to the normal population below 8 years old and to their siblings in the group between 4 and 8 years old (p < 0.05).
DISCUSSION
IgG subclasses have biological properties with intrinsic variances. The complement fixation, placenta crossing, receptor binding, antigen binding and functional valence are different in the four different subtipes of IgG (1). Several studies have established an association between the deficiency of certain IgG subclasses and the predisposition for recurrent infections of the respiratory airways (6). The antibodies for virus are specially IgG1 and IgG3 associated subclasses. The response to bacterial polysaccharide antigens is predominantly an IgG2 and IgG4 reaction (2).
More than to define structural differences, it is important to consider the biological dynamics of each subclass and how this deficiency is clinically expressed. In fact, the deficiency of one or several subclasses can be conditioning factor of an immunological system incapable to answer adequately to patogenic aggressions. In the other hand, there are several reports of individuals with IgG subclass-deficient profile without abnormal number of infections (4). This is due probably to conpensatory immunological mechanisms present in these subjects not yet well studied. Recent studies emphasized that there is a lack of correlation between the level of IgG subclasses and the individual susceptibility to infections, claiming more importance for the antibody response to certain antigens (4).
However, several studies referred that those patients with respiratory Haemophilus influenzae infectious processes are more prone to have IgG deficiencies than individuals with other types of infectious diseases (1, 4, 7, 8). The IgG4 deficiency is the most common in this group. Several other studies tried to establish a correlation between the occurrence of specific IgG subclass and infectious pathology. In any case, the reported incidence of IgG subclass deficiencies vary considerably between the published studies, often limited by different methodological approaches (4).
The subclass IgG2 and IgG4 antibodies have been associated with the protection against infections by capsulated bacterial microorganisms, such as Streptococcus pneumoniae and Haemophilus influenzae (2). Particular emphasis has been placed in the role played by IgG4 (4). Diverse authors have enhanced the importance of the IgG4 in the lung and mucosal immunological defenses. Although IgG4 is minor subclass (2-6 % of total IgG) and lack some of the properties of their IgG counterparts, it may play a not yet correctly defined role in host immunological defense. It also acts as a marker for restrictions in the production of IgG2 antibodies for Staphylococcus aureus, even with IgG2 normal values. A number of studies reported also limitations on the IgG1 response to Haemophilus influenzae immunization in cases where IgG2 low levels were identified (4, 7).
Total IgG concentration should not be a form to identify IgG subclass deficiency, mainly because the IgG2 and IgG4 represent a small ratio of the total IgG. In children the concentrations of IgG subclasses are lower, requiring quantification with methods that are more sensible. In our sample, from thirty seven children with low IgG4 levels, only in two cases were simultaneously identified lower concentrations of total IgG concentration. In Down syndrome, this is more pertinent attitude since this population was associated with lowest IgG2 and IgG4 values. The upper values of IgG1 and IgG3 observed in our sample can be a consequence of the polyclonal stimulation caused by recurrent bacterial infections. However, the determination of the number of infectious episodes was assumed as a difficult task to fulfill in ambulatory, due the clinical subtlety of many of these.
The definition of the underlying mechanisms of IgG2 and IgG4 deficiency has been a controversy point (9). In our sample in more than half of the pairs of brothers T21-FT21 was verified and agreement in the reduction of the IgG4 in both groups (T21 and FT21). In children with the same parenteral origin, our results point out for the interest of IgG subclass quantitative study in children with SD and their siblings.
From our data is possible to substantiate that siblings from T21 children have a tendency to vary their total IgG and subclasses serum concentrations in the same manner. Is not clear how the genetic background could contribute as one of the responsible factors. There is a potential explanation arising from the possibility of T21 could act as infectious vectors to their family counterparts, influencing directly their immune profile. Preventive measures to minimize infections in T21 children could improve favorably their brotherhood.