INTRODUCTION
Pollen from Parietaria judaica (Pj) is a major source of airborne allergens in the Mediterranean area, giving rise to seasonal allergies with clinical manifestations such as rhinitis, conjunctivitis and asthma. Profilins constitute a ubiquitous family of proteins that control actin polymerization in eukaryotic cells (1) and have been identified as allergens in several species of tree, grass, and weed pollens, and in some fruits and vegetables. Profilins are described as pan-allergens, because they are recognized by 20 % of all pollen-allergic patients (2-4), and have been considered responsible for combined allergies to pollen and food (4).
It is well established that allergen-specific TH2-like cells play an important role in the induction of IgE synthesis (IL-4) and eosinophilia (IL-5) (5-7). Furthermore, a higher frequency of infiltrating T-cells producing TH1 cytokines (IFN-γ) (8-10) and an increase in allergen-specific IgG (11) are found after successful immunotherapy. This stresses the importance of different subsets of T-cells in the allergic immune responsiveness. Whereas skin prick test (SPT) areas and allergen-specific IgE measurements are well correlated (12, 13), no relationship between allergen-specific T-cell proliferation, cytokine production, allergen-specific IgE, and SPT, has been found (14).
In this study we examine the specific immune response to Pj plant profilin in atopic patients and non-atopic subjects. We analyze the possible correlation between allergen-specific T-cell proliferation, cytokine production, allergen-specific IgE and SPT in atopic patients to Pj profilin and non-atopic donors. In addition, we address the question of the cross-reactivity between Pj and Phl p profilins.
METHODS
Characterization of blood donors
Blood samples from 28 Pj profilin allergic patients (mean age 32 years, range 19-54) and 18 non-atopic subjects (mean age 39 years, range 25-53) were collected. All patients had a clinical history of seasonal allergic rhinitis and/or asthma, positive SPT (> 4 mm of wheal diameter) to Pj pollen extracts and Pj profilin, and all but one had positive serum Pj-specific IgE (CAP-System, Pharmacia, Uppsala, Sweden) levels in serum. None of the patients had received specific immunotherapy. Histamine chloryde, 10 mg/ml and saline were used as positive and negative controls, respectively, in all subjects.
None of the control donors had a clinical history of allergic disease, and none had specific serum IgE or a positive SPT reaction to any of the common allergens of the Mediterranean area.
Allergens
Pollen from Pj (Allergon AB, Välinge, Sweden) was extracted as previously described (15) and stored lyophilized at 20 °C until used. Working solutions (1 mg lyophilized material/ml RPMI 1640 medium (BioWhittaker, Walkersville, Maryland) were stored at 20 °C until used. The extracts contained all important allergens as evaluated by sodium dodecylsulfate-polyacrylamide gel electrophoresis immunobloting. Chromatographyc purification of profilins was done as previously described (16). Proteins co-eluted with profilin were removed by high-resolution chromatography in the SMARTTM system (Pharmacia), as also described (16).
PBMC stimulation assay
PBMCs were isolated from freshly drawn heparinized blood by gradient centrifugation on Lymphoprep (Nycomed Pharma, Oslo, Norway), washed twice with PBS (bioMérieux, Marcy l'Etoile, France), and resuspended in X-VIVO 10 medium (BioWhittaker, Walkersville, Maryland) with L-glutamine, without antibiotics (referred to as medium). The PBMCs were stimulated with 10 mg/ml of Pj profilin, in 96-well, round-bottomed plates with 1 X 105 cells/well in 200 μl medium/well. Optimal conditions for the proliferation assays were determined by pilot studies with allergen concentrations from 100 to 0.01 μg/ml and harvest of the cells at day 3, 6 or 10 with PBMCs from patients and non-atopic donors. PBMCs in cultures without allergen were used as negative controls; PBMCs stimulated with 5 mg/μl tetanus toxoid (TT) (bioMérieux, Lyon, France) or with 1/100 PHA (Wellcome, Beckenham, U.K.) were used as positive controls. Cells were cultured for 6 days in a humidified atmosphere at 37 °C, 5 % CO2, followed by an 18-hour pulse in the presence of 0.5 μCi of 3H-thymidine (Amersham, U.K.) per well. The cells were harvested on to nitrocellulose filters and thymidine incorporation was determined by liquid scintillation counting (Betaplane, BTK, Wallac). Stimulation index (SI) values were calculated as the ratio of cpm at the optimal antigen concentration in the stimulated cultures relative to the cpm in the unstimulated cultures. All results represent the mean values of triplicate cultures.
Cytokine production
PBMCs were thawed and stimulated in medium with 25 μg/ml of Pj profilin or 1/100 PHA (Wellcome, Beckenham, U.K.), in 96-well, flat-bottomed plates; 3 x 105 cells/well in 300 μl medium/well were used. The cells were cultured in humidified atmosphere at 37 °C, 5 % CO2; PHA and Pj profilin supernatants were collected at days 2 and 7 respectively. IL-4 and IFN-γ concentrations in supernatants were determined by ELISA (Bender, Vienna, Austria) according to the manufacturer's description.
Establishment of Pj specific T-cell lines
T-cell lines specific to Pj profilin were established from PBMCs of 27 patients allergic to this profilin. Isolated PBMCs (1 x 106/ml) were stimulated in 8 x 1 ml bulk cultures in 24-well flat-bottomed culture plates (Nunc, Roskilde, Denmark) with 10 mg/ml of profilin for 7 days. Subsequently, recombinant IL-2 (rIL2) (10 U/μl; Roche, Nutley, New Jersey, USA) was added and kept in culture for an additional 7 days.
T-cell stimulation assay
On day 14 after stimulation, the proliferative capacity of the T-cell lines was assessed: 2 x 104 T-cell blasts were incubated in triplicate in 200 μl cultures in the presence of 105 irradiated (2,500 rads) autologous PBMCs plus the appropriate profilin (10 μg/ml) in 96-well round bottomed microtitter plates for 48 hr at 37 °C, in a humidified atmosphere of 5 % CO2, followed by an 18-hour pulse with 0.5 μCi of 3H-thymidine per well (Amersham), radionuclide uptake was measured by scintillation counting. Cultures with positive SI were selected.
Statistical analysis
Comparison of T-cell proliferation (expressed as SI) and cytokine production (expressed as pg/ml) between groups was done by Student's t test or Mann Whitney U test to compare groups of data with non-normal distribution. Spearman rank order correlation was used to detect correlation between different data sets. All stochastic probabilities less than or equal to 0.05 were considered significant. Analyses were performed with SPSSWIN 6.1.3 (SPSS Inc., 1989-1995).
RESULTS
SPT and allergen-specific IgE
All patients had a positive SPT to both Pj crude extract and Pj highly purified profilin, and all but one had Pj specific IgE. No correlation was found between the patients' SPT wheal diameter (mean 7,75 ± 2,2 SD) and the concentration of specific IgE in serum (fig. 1). Total serum IgE levels ranged from 11 to 1,511 IU/ml (mean 228 ± 316 SD). Pj specific IgE levels range from 0.3 to 97 KU/L (mean 28.9 ± 27.2 SD).
Figure 1.--SPT versus specific IgE. SPT results are expressed as Pj profilin wheal diameter in mm. Pj-specific IgE concentrations were determined in a CAP-SYSTEM. (Pharmacia, Upsala, Sweden) and expressed as KU/L. Spearman rank order correlation: r = 0.04, p = 0.82.
Proliferative T-cell responses to allergens
Both patients and control subjects exhibited proliferative T-cell responses to the allergen extract with maximal stimulatio ranged 1 to 100 μg/ml. To ensure that the results were not influenced by the variation in background counts, they were expressed as SI. The responses expressed as mean SI to crude extract and highly purified profilin were higher in the group of atopic patients than in the non-atopic subjects (fig. 2). Proliferative responses of patient's PBMC to Pj profilin were generally low, with SI values ranging from 1 to 6 (mean 2,04 ± 0.7 SD). This finding was in contrast with their SPT response to Pj profilin and high Pj specific IgE. Proliferative responses to TT and PHA were higher in the group of non-atopics than in patients, but the difference was not significantly (fig. 2).
Figure 2.--Specific T-cell responses to profilin and crude extract from Pj. Results are expressed as SI obtained by stimulation of PBMCs from atopic donors (closed figures) or non-atopic controls (open figures). Mean values are significantly higher for Pj profilin in atopics (mean 2.04 ± 0.7 SD) than in controls (mean 1.47 ± 0.28 SD) (p = 0.007). Also, mean values are significantly higher for Pj crude extract in atopics (mean 3.47 ± 2.5 SD) than in controls (mean 1.17 ± 0.16 SD) (p = 0.003). Mean values are non significantly higher for TT in controls (mean 10.5 ± 4.2 SD) than in atopics (mean 5.7 ± 3.9 SD) (p = 0.481). Also, mean values are non significantly higher for PHA in controls (mean 152 ± 60.7 SD) than in atopics (mean 121 ± 59 SD) (p = 0.98).
To further analyze the Pj profilin-specific T cell reactivity 185 T cell lines were generated from the peripheral blood of 27 Pj profilin allergic patients. A substantial increase in the SI was achieved, but the majority of T cell lines still exhibited low reactivity against Pj profilin (median 4 ± 6.2 SD).
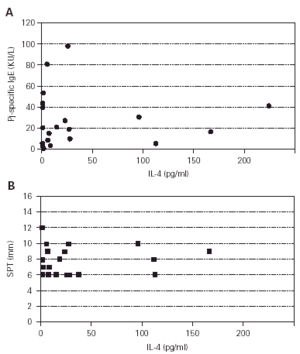
The level of profilin-specific T-cell proliferation did not correlate with specific Pj IgE concentrations (fig. 3A) or Pj profilin SPT (fig. 3B) of the individual patients.
Figure 3.--(A). Specific T-cell responses to Pj profilin versus specific IgE. Results are expressed as SI obtained by Pj profilin stimulation of PBMCs from atopic donors and Pj-specific IgE (KU/L). The IgE concentrations were determined in a CAP-SYSTEM (Pharmacia, Uppsala, Sweden). Spearman rank order correlation: r = 0.16, p = 0.432. (B). Specific T-cell responses to Pj profilin versus SPT. Results are expressed as SI obtained by Pj profilin stimulation of PBMCs from atopic donors and Pj profilin wheal diameter in mm. Spearman rank order correlation: r = 0.09, p = 0.620.
Cytokine profiles of the allergen-specific T-cell response
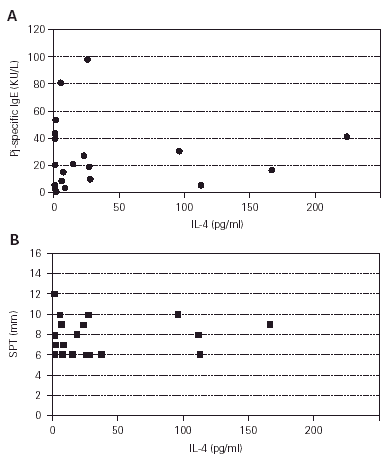
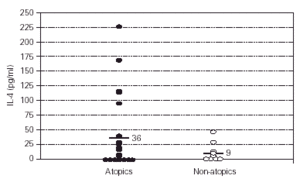
To determine whether the cytokine production of the patients was consistent with the atopic phenotype, supernatants from profilin and PHA-stimulated PBMCs from patients and control subjects were assayed for production of IL-4 and IFN-γ. The production of IL-4 on PHA-stimulated PBMCs from atopic patients was higher than from control subjects (p = 0.001) (fig. 4), whereas the production of IFN-γ on the same conditions was not different (p = 0.38, results not shown). The production of IL-4 and IFN-γ by profilin-stimulated PBMCs was undetectable in the majority of patients and controls (results not shown). IL-4 polyclonal production level did not correlate with the specific Pj IgE concentration (fig. 5A) or Pj profilin SPT (fig. 5B) in the atopic patients.
Figure 4.--IL-4 production by PBMCs of atopic patients (closed circles) versus non-atopic donors (open circles). Polyclonal cytokine production: PBMCs were incubated with PHA (1/100) for two days and the concentration of IL-4 was determined by ELISA. Atopic patients produced higher levels of IL-4 (mean 36 ± 59 SD) than non-atopic controls (mean 9 ± 15 SD) (p = 0.001).
Figure 5.--Correlation between polyclonal IL-4 production and specific IgE (A) or SPT (B). Pj-specific IgE concentrations were determined in a CAP-SYSTEM. (Pharmacia, Upsala, Sweden) and expressed as KU/L. The concentration of IL-4 was determined by ELISA, and expressed as pg/ml on the supernatants of the cultures of PBMCs from Pj atopic patients stimulated with PHA (1/100) for two days. The Pj profilin wheal diameter are expressed in mm. Spearman rank order Correlation: A: r = 0.003. B: r = 0.161.
Cross-reactivity of Pj and Phleum pratense profilins
To study the possible T-cell cross-reactivity we selected 7 of the better Pj profilin T-cell lines stablished from the PBMCs of four patients. This T-cell lines did not show cross-reactivity with another highly purified profilin (from Phl p) (fig. 6).
Figure 6.--Reactivity of Pj profilin specific T-cell lines with a highly homologous profilin from Phl p. Seven T-cell lines were established from the PBMCs of four Pj profilin atopic patients. Results are expressed as the mean of cpm of triplicate cultures, stimulated with Phl p profilin (mean 2045 ± 1433 SD), Pj profilin (mean 10269 ± 5477 SD), or medium alone (mean 2207 ± 2395 SD).
DISCUSSION
The main findings of our study are: (a) The patients allergic to Pj profilin had a significantly increased T-cell proliferative response to this profilin compared with non-atopic subjects. (b) The enhanced T-cell proliferation of the atopic patients was not correlated to skin reactivity or to specific serum IgE levels. (c) Specific Pj profilin T-cell lines did not show cross-reactivity with another highly purified profilin (Phl p profilin). (d) The group of atopic patients has a significantly increased polyclonal (PHA) production of IL-4, compared with non-atopic subjects, and (e) this increased production of IL-4 did not correlate with skin reactivity or specific serum IgE levels.
The enhanced T-cell responses to profilins in the atopic patients, although small, indicate an increased frequency of allergen-specific T-cells (17). The fact that enhanced profilin specific T-cell responses are found in the peripheral blood of patients suggests that the responding T-cells are inducing the production of the allergen-specific IgE by B cells and the subsequent skin reactivity. Therefore, a direct correlation between the level of T-cell reactivity and allergen-specific serum IgE and SPT could be expected. However, in this study neither specific serum IgE levels nor SPT diameters could be correlated to the level of profilin specific T-cell responses of the patients. In similar studies, we (18) and others (14), working with Phl p profilin and grass allergen extracts and immunoaffinity-purified group 5 allergens of the Poaceae family (Phl p, Poa pratensis, Lolium perenne) reached similar conclusions. In these works, neither it was found any correlation between the magnitude of the patients' T-cell responses and the skin prick test areas or specific serum IgE levels.
The small increase in the T cell response of the patients group, was in contrast to the positive results of the SPT, that were in general vigorous. Low responsiveness in the proliferation test were characteristics of Pj profilin when assayed in PBMCs and also with T-cell lines. A general defect in the proliferative capacity of lymphocytes in these patients could be excluded because the TT and PHA controls induce good proliferative responses. Similar low T cell responsiveness has been described with Bos d 2 (19), s1-casein (20) and Fel d1 (21). The explanation of how a low T-cell responsiveness can sustain and elevated SPT in the group of Pj profilin atopic patients, remains especulative at this moment, but could be related to the fact that profilins are ubiquous and highly conserved proteins: there is a 70 % of aminoacid sequence homology between human and plant profilins.
We were unable to detect the production of IL-4 (a typical TH2 cytokine) and IFN-γ (a typical TH1 cytokine) when PBMCs from atopic patients and non-atopic controls were stimulated by Pj profilin because the levels of the cytokines in the supernatants of the cultures were too low for the sensitivity of the method. However, the increased production of IL-4 and the tendency to a reduced production of IFN-γ, due to a polyclonal stimulation (PHA) of peripheral T-cells of the atopic patients, reflects their allergic phenotype. Several researchers have reported cytokine production patterns in PBMCs activated by non-specific stimulation in atopic diseases. It has been shown that in vitro IFN-γ production by PHA-stimulated PBMCs is greatly diminished in atopic dermatitis patients compared with non-atopic healthy controls (22). In addition, it has also been demonstrated that the activation of PBMCs from atopic patients with high serum IgE induce higher levels of IL-4 synthesis and lower levels of IFN-γ than the levels produced by PBMC from healthy controls, suggesting that enhanced IL-4 production and reduced IFN-γ production are associated with the elevated serum IgE levels in atopic patients (23, 24).
Recently, several plant profilins have been cloned and sequenced (25-29). Their deduced amino acid sequences show a high degree of homology. However, the T-cell lines raised against Pj profilin did not show any reactivity with Phl p profilin. Even though these data were obtained from experiments with a limited number of T-cell lines, the results indicate the importance of using T-cell lines (or clones) and highly purified (or recombinant) allergens in order to stablish the cross-reactivity between allergens at the T-cell level.
In conclusion, the results of the present study show that Pj profilin specific proliferative T-cell response and the polyclonal production of IL-4 are higher in patients than in the group of control subjects. This suggests a role for Pj profilin in the induction of T-cell-driven immunological reactions. The lack of correlation between the skin reactivity and IgE-specific concentration, T-cell reactivity or IL-4 production most likely reflects the complexity of the skin reactivity, whereas the fact that the proliferative T-cell response did not correlate to the patients serum IgE suggests that at least some of the responding T-cells may be involved in immune reactions other than the support of IgE production. That profilins are ubiquous and highly conserved proteins could be the explanation of the low T-cell responsiveness and elevated SPT, in our group of patients. Pj profilin specific T-cell lines did not show cross-reactivity with a highly homologous profilin from Phl p (14).
ACKNOWLEDGEMENTS
This study was supported by FISS 96/0787. The hrIL-2 used in this study was kindly provided by Dr. M. Gately (Hoffmann-La Roche, Nutley, New Jersey, USA).
Abbreviations used
SPT: Skin prick test
PBMC: Peripheral blood mononuclear cells
PBS: Phosphate-buffered saline
PHA: Phytohemaglutinin
TT: Tetanus toxoid
SI: Stimulation index
Pj: Parietaria judaica
Phl p: Phleum pratense