INTRODUCTION
Clopidogrel, an oral antiplatelet drug, has significantly reduced event rates after percutaneous intervention, and has improved long-term outcomes. Due to the inhibition of platelet aggregation via adenosine diphosphate-dependent mechanisms, clopidogrel has become the most important medication after coronary stent implantation. In a small percentage of patients taking this medication, adverse effects can include rash, which is not sufficiently severe to require discontinuation of the drug. In the event clopidogrel cannot be used, alternative treatments such as ticlopidine offer lesser efficacy and require frequent follow-up and testing to prevent even more serious adverse effects.
CASES REPORT
Case 1
A 58-year-old male presented with a history of ischemic heart disease (non-Q-wave myocardial infarction, single-vessel coronary disease with preserved systolic function, with successful stent implantation in an obtuse marginal branch) and renal colic. During his stay in intensive care, the patient presented erythematous and pruriginous symptoms on both elbows and arms that persisted for 15 days despite medication with corticoids and antihistamines. The symptoms disappeared when clopidogrel was stopped. The base treatment up to that time comprised omeprazole, acetylsalicylic acid, clopidogrel (Plavixì), enoxaparin, ramipril, atenolol, simvastatin, lactulose, and bromazepam. There were no family antecedents from the allergic point of view.
Allergy tests: Skin prick-test on the inner side of the forearm with clopidogrel at a concentration of 7.5 mg/ml (1:10), considering as positive the appearance of a papule and erythema of similar size to the histamine control in 20 minutes. The test proved positive, with the appearance of a papule measuring approximately 4 mm with regard to the negative control.
The prick-test proved negative in a control group of 5 patients who had received clopidogrel previously.
Diagnosis: Immediate reaction after clopidogrel intake, in which we demonstrated hypersensitivity to clopidogrel through cutaneous tests that suggest a type 1 hypersensitivity mechanism.
Case 2
An 81-year-old male presented with a history of ischemic heart disease, ventricle dysfunction, clouding of the lens of the eye, arthrosis, hyperlipidemia, and right intraparenchymal hemorrhage. Twenty-four hours after cardiac catheterization, he developed generalized pruritus, erythema and desquamation. The medication up until that time consisted of clopidogrel (Plavix®), acetylsalicylic acid, omeprazole, and simvastatin. The rest of the allergic questionnaire proved negative
The symptoms persisted approximately two months, and the patient general conditions improved after the substitution of clopidogrel with heparin sodium.
Allergy skin tests: We started carrying out a prick-test on the inner side of the forearm with clopidogrel at a concentration of 7.5 mg/ml (1:10), considering as positive the appearance of a papule and erythema of similar size to the histamine control in 20 minutes.
As the prick-test proved negative, an intradermal test was performed with a 1:10 dilution of the concentration used in the original prick. A positive reaction was defined by an increase in the papule beside erythema similar to the positive histamine control. In both the prick and intradermal tests, the readings were taken after 20 minutes and after 8, 48 and 72 hours. Also, this test proved negative.
In short, both the prick-test and the intradermal reaction proved negative for clopidogrel.
The medication used proved negative in a control group of 5 individuals previously administered clopidogrel.
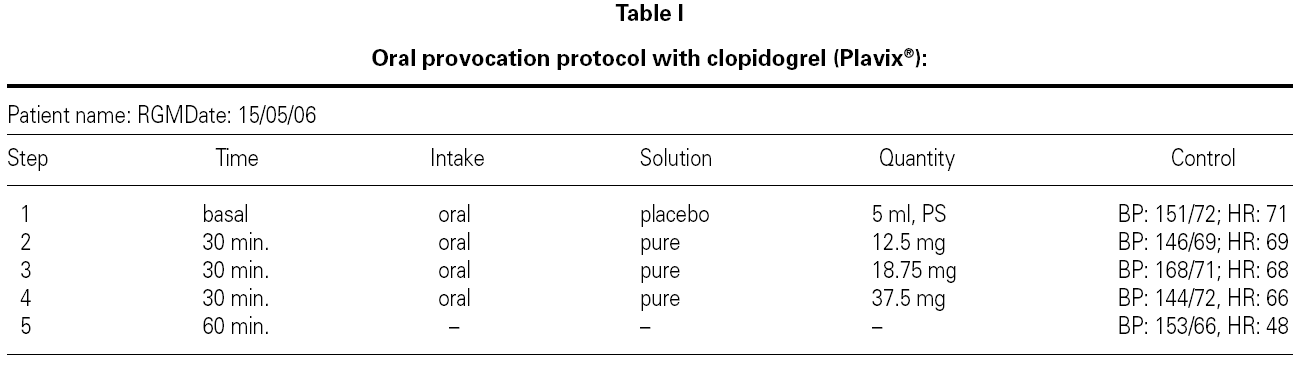
Oral challenge tests: Were performed in a placebo-controlled, single-blind manner with clopidogrel, resulting in good immediate tolerance, though after 24 hours the patient developed generalized and pruritic erythema that subsided with oral treatment (corticoids and antihistamines).
Posteriorly, a single-blind placebo-controlled oral challenge test was performed with ticlopidine, revealing good immediate and time-delayed tolerance.
Epicutaneous tests of clopidogrel (mixed with 30 % vaseline) with readings after 48 and 72 hours were negative.
Diagnosis: Non-immediate allergic reaction to clopidogrel.
DISCUSSION
Hypersensitivity reactions are immunologically-mediated reactions caused by exposure to an antigen. Reported clinical presentations of clopidogrel hypersensitivity include urticaria1, skin rashes2-4 and angioedema5. While the precise immunologic mechanism of clopidogrel hypersensitivity has not been elucidated, the report of a patient with positive intradermal skin testing6 would support an IgE-mediated process. Other potential mechanisms for such hypersensitivity reactions would include direct mast cell activation, complement activation, immune complex formation, T-cell activation, or other unknown mechanisms. It is also possible that the hypersensitivity reactions could be caused by a clopidogrel metabolite rather than directly by clopidogrel itself.
The consulted literature describes some desensitization protocols for clopidogrel, but we have found no reports of allergic testing to elucidate the immunologic mechanism involved (table I).
We have described two hypersensitivity reactions due to clopidogrel. The first case corresponded to a patient who presented a compatible episode with immediate reaction after clopidogrel intake, in which hypersensitivity to the drug was demonstrated by cutaneous tests suggesting a type 1 hypersensitivity mechanism. The second patient suffered a late reaction due to type 4 sensitization to clopidogrel, confirmed by oral provocation, where good tolerance to other antiplatelet drugs such as ticlopidine was demonstrated.
Correspondence:
Dr. Diego Gutiérrez-Fernández
Avda. Constitución, 3, 3G
11160 Barbate (Cádiz). Spain
Fax: 956 00 29 21
Telf.: 639307042
E-mail: drgutierrez@comcadiz.com