In Spain, the indications for the triple viral vaccine and antiinfluenza vaccine are established by the Vaccines Advisory Committee (VAC) of the Spanish Pediatrics Association. The calendar recommended for this year (2007) establishes a first triple viral vaccine dose at 12-15 months of age, followed by a second dose at 3-4 years of age. The indications for the antiinfluenza vaccine in the pediatric population have increased in recent years. Accordingly, among the recommendations for the campaign 2006-2007, the VAC included antiinfluenza vaccination of children with chronic illnesses, including respiratory disorders and specifically bronchial asthma1. In the United States, the indications of the Advisory Committee on Immunization Practices (ACIP) for this same vaccination campaign moreover included healthy children between 6-59 months of age2.
On the other hand, a continuous increase in atopic disorders is being observed, including food allergies, which affect 8 % of the pediatric population. Allergy to egg is the most common food allergy in this age group, and in 79 % of cases the disorder manifests before 5 years of age3. Thus, an increasing number of children with egg allergy are seen in which administration of the triple viral vaccine and antiinfluenza vaccine is indicated.
In view of this problem, the Food Allergy Committee of the Spanish Society of Clinical Immunology and Pediatric Allergy (Sociedad Española de Inmunología Clínica y Alergia Pediátrica, SEICAP) has examined the subject with the purpose of defining a series of recommendations or guidelines.
TRIPLE VIRAL VACCINE
In Spain, two vaccines against measles, mumps and rubella (MMR triple viral vaccine) are marketed: Triple vaccine MSD® (Sanofi Pasteur MSD) and Priorix® (GSK). These vaccines are widely used in the pediatric population.
Two of the components of this vaccine (measles and mumps) are obtained from attenuated live viruses cultured in chicken embryo fibroblasts hence concern over the possible presence of egg proteins in the formulation and their administration to patients with egg allergy. The attenuated rubella virus is obtained from human diploid fibroblast cultures.
Up until the year 2004, and despite the improbability of any serious allergic reaction after conventional triple viral vaccination of an individual with egg allergy alternative vaccines being available in Spain (Triviraten® and Moruviraten®) the use of such alternatives was recommended despite the lesser immunogenicity and vaccination coverage afforded (Moruviraten®).
Individuals with a history of anaphylactic reactions after egg consumption were considered to be at risk after the administration of vaccines obtained from cultured chicken embryo fibroblasts. Current evidence suggests that anaphylactic reactions secondary to mumps and/or measles vaccination are not associated with egg antigen hypersensitivity but with other components of the vaccine (such as gelatin or neomycin)4,5. Analyses of the amounts of egg protein have been made using different techniques, and this may account for the range of results obtained from non-detection to amounts of 0.5 to 1 ng of ovoalbumin per 0.5 ml dose of vaccine6-8. The gelatin content is 14.5 mg, and that of neomycin sulfate 25 μg, per vaccine dose. Accordingly, the greater amounts of these allergens make them more likely candidates for allergic reactions than egg protein 9.
Since the 1960s, articles have been published on the safety of the triple viral vaccine in patients with egg allergy. In 1995, James conducted a review of the literature on the subject10. Out of a total of 1265 evaluated patients, including 1237 with positive skin testing for egg (out of 1247 tested individuals) and 284 positive oral provocations for egg (out of 292 tests performed), only two vaccination reactions were reported out of 1227 patients administered the vaccine as a single subcutaneous dose (patients without skin testing for egg or oral provocation testing). Recent studies in the Spanish pediatric population likewise confirm the safety of the vaccine in patients allergic to egg11,12.
In the different studies found in the literature, skin testing with the triple viral vaccine yields poor positive and negative predictive values for assessing the risk of allergic reactions after administration, and moreover systemic reactions have been described during the diagnostic process. Desensitization has also been attempted to prevent allergic reactions to the vaccine in individuals with egg allergy though little evidence can be found in the literature in support of this approach. The risk of serious allergic reactions after triple viral administration is extremely low, and skin testing with the vaccine is not predictive of allergic reactions after vaccination4,5,10,13,14.
The guide published in 2000 by Khakoo recommended vaccination at hospital level among patients with egg allergy who present persistent bronchial asthma with the need for background medication, on the grounds of the increased risk of anaphylactic reactions to foods in these patients13-15. However, the current guidelines do not include this measure of caution1,4,5,16.
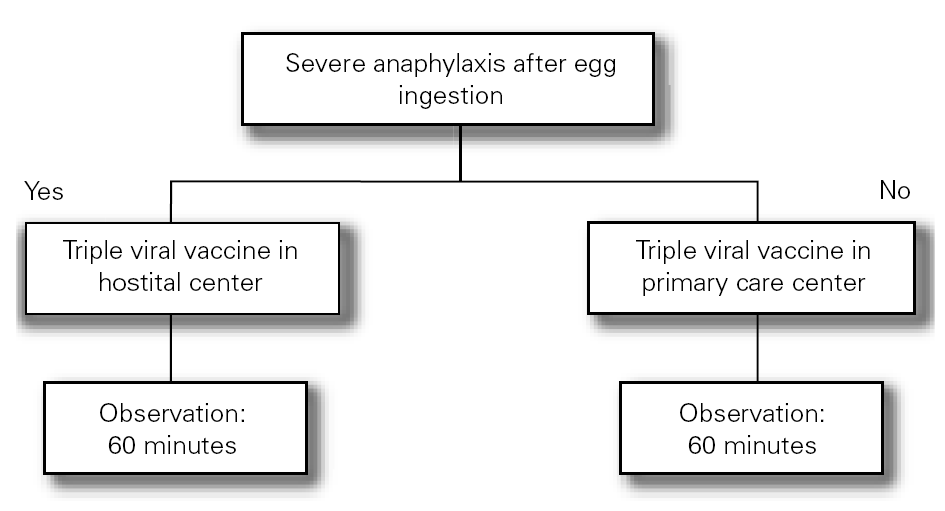
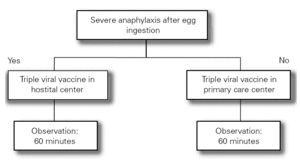
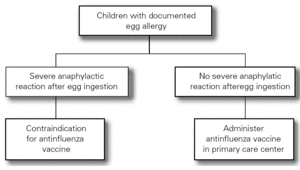
The Food Allergy Committee of the SEICAP recommends the following (fig. 1):
Figure 1:--Triple viral vaccine.
1.All children with egg allergy can be vaccinated in their vaccination center with the usual triple viral vaccine, and should remain in the center for 60 minutes after vaccination.
2.Only in the case of those children with serious anaphylactic reactions after egg consumption is vaccination recommended in a hospital center.
3.The vaccine is contraindicated in the case of serious anaphylactic reaction to a previous vaccine dose or to its components (gelatin, neomycin, etc.). The rest of general contraindications to the vaccine also apply.
ANTIINFLUENZA VACCINE
The two types of antiinfluenza vaccine currently available (inactivated trivalent vaccine and attenuated live virus vaccine) are obtained from embryonated chicken eggs, and the vaccines contain egg proteins. The inactivated virus vaccine is marketed and authorized for pediatric use in Spain. The section relating to contraindications in the Summary of Product Characteristics of these vaccines cites hypersensitivity to egg or to any of the product components, without further specification.
Different studies have quantified the amounts of egg protein in the antiinfluenza vaccine, with highly variables results. Thus, ovoalbumin ranges from undetectable levels to 42 μg per ml of vaccine17,18. These amounts are far higher than those found in the triple viral vaccine, and are sufficient to pose a risk for patients with allergy to egg.
Unlike the recommendations for the triple viral vaccine, which can be safely administered to patients with egg allergy (including those with antecedents of anaphylactic reactions to egg), the administration of the antiinfluenza vaccine in such children requires certain considerations and measures of caution, since the vaccine may contain significant amounts of egg protein.
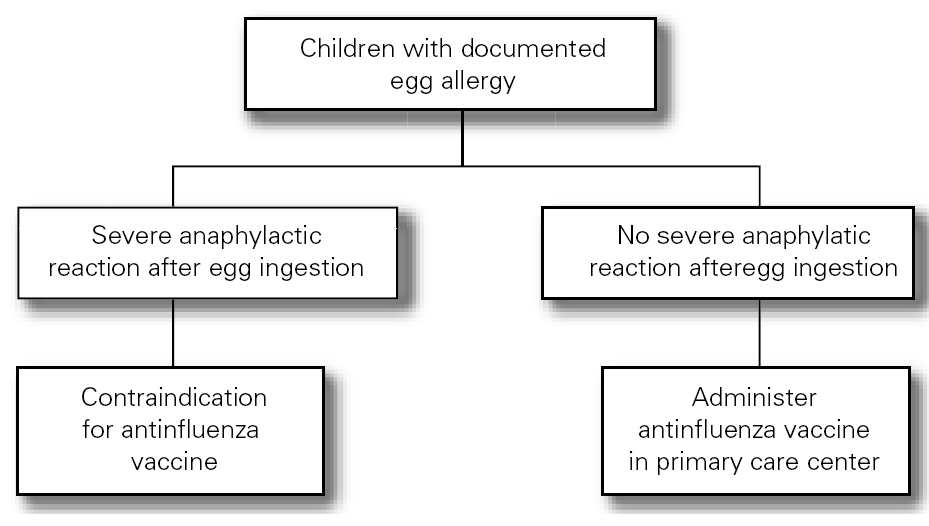
The antiinfluenza vaccine can be administered to patients with egg allergy, except when a serious allergic reaction (anaphylaxis) is observed after egg consumption in which case vaccination is contraindicated2,19,20.
It must be taken into account that the only large safety study of triple viral vaccination among individuals with egg allergy (n = 83) was carried out with vaccines containing ≤ 1.2 μg of egg protein per ml of vaccine, and the latter was moreover administered in two doses spaced 30 minutes apart (first dose: 1/10 of the vaccine; second dose: 9/10 of the vaccine). One-third of the patients included in the study presented anaphylactic symptoms after egg ingestion. None of the patients suffered an allergic-type adverse reaction. The patients requiring a second vaccination dose after one month received the full dose without problems18.
The current recommendations for antiinfluenza immunization include asthmatic children. Although the 2000 Cochrane review stated that the studies conducted to date in relation to safety in asthmatic patients are inconclusive, posterior studies have warranted the safety of vaccination and the absence of any association with asthma exacerbation21.
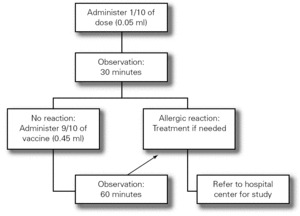
The Food Allergy Committee of the SEICAP recommends the following:
1.The antiinfluenza vaccine is contraindicated in those children with serious anaphylactic reactions after egg consumption or following a previous antiinfluenza vaccine dose (fig. 2).
Figure 2.--Antiinfluenza vaccine.
2.Use should be made of vaccines containing ≤ 1.2 μg of egg protein per ml of vaccine.
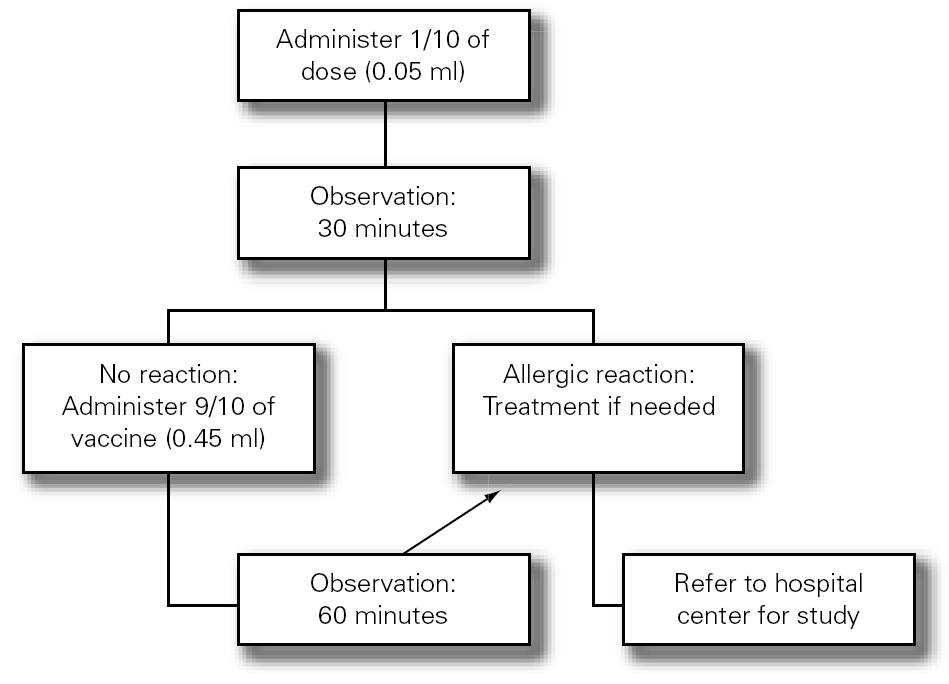
3.Fractionated dosing is indicated: first dose equivalent to 1/10 of the vaccine, followed 30 minutes later by the rest (9/10 parts). The patient should be kept under observation during one hour (fig. 3).
Figure 3.--Antiinfluenza vaccination protocol.
4.If a second dose proves necessary after one month, and provided there have been no contraindicating reactions, the former may be provided as a single dose again keeping the patient under observation during one hour.
To facilitate the choice of antiinfluenza vaccine, the Food Allergy Committee has contacted the different laboratories to determine whether they quantify ovoalbumin, and whether it is possible to know the amounts contained in the different batches. The replies have been the following:
Leti polyvalent fractionated antiinfluenza vaccine®, Pasteur antiinfluenza vaccine® (Sanofi Pasteur MSD) and Berna Biotech Inflexal V® report compliance with the European Pharmacopoeia, and state that all marketed batches contain ovoalbumin in amounts of ≤ 0.1 μg/ml of vaccine.
GSK Fluarix® contains no more than 1 μg of ovoalbumin per dose (0.5 ml).
Esteve Chiroflu® states the presence of egg protein, but not the amounts.
Solvay Pharma Influvac®: ovoalbumin contain is 2 or less μg/ml of vaccine.
Correspondence:
Dra. Mónica Piquer-Gibert
Sección Inmunoalergia
Hospital Sant Joan de Déu
Sant Joan de Déu, 2
08950 Esplugues (Barcelona). Spain.
E-mail: mpiquer@hsjdbcn.org