INTRODUCTION
Severe IgE-mediated sensitivity to peanut and other legumes, can pose lifelong difficulties in dietary management for some patients involving marked limitations in their lifestyle. The clinical manifestation of sensitivity can occur on first exposure. In most of such cases the likely route of sensitisation is through breast milk 1,2. A history of sensitivity shows good correlation with the results of RAST and skin prick testing 3-5. Peanut sensitivity can persist throughout life and it is well documented as a cause of life-threatening anaphylaxis 6. Yunginger et al 7 reported 7 incidences of fatal food-induced anaphylaxis. Peanut was implicated as the responsible food in four of the cases.
Bernhisel-Broadbent and Sampson 8 investigated cross reactivity among legumes. They suggested that cross reaction is uncommon in double blind, placebo controlled, food challenges despite positive RAST and skin prick tests to several members of the legume family. However, in those patients who exhibit anaphylaxis to peanut and where cross reaction is confirmed on challenge, strict dietary avoidance is essential and forms the mainstay of long-term management. Inadvertent ingestion can often occur due to the common presence of legumes and legume-derived emulsifiers in processed foods. The digestibility of legume proteins is poor due to difficulties of hydrolysis by gastrointestinal enzymes and the presence of specific enzyme inhibitors 9. This may partly account for the common occurrence of legume sensitivity amongst the atopic population. We have suggested that the major protein fractions of legumes may have comparable allergenic potency and the clinical effect depends upon the combination of allergenicity and the quantity presented to the sensitised patient's immune system 10.
Shenassa, Perelmutter and Gerrard 11 described the experience of 6 patients using an oral desensitising program for peanut. Four of the patients reached a stage of tolerating peanut daily. The degree of sensitivity to other legumes was not reported and the IgE and IgG4 RAST results were not given. Carlston 12 outlined the use of parenteral peanut immunotherapy in a patient with rhinitis symptoms triggered by the odor of peanuts and peanut butter. After immunotherapy, the patient developed improved tolerance to the inhalant effects, but there was no reference to any benefits following ingestion. Oppenheimer, Nelson, Bock et al 13 reported the treatment of peanut allergy with rush immunotherapy. Unfortunately, the study was not completed because of a fatal incident caused by the administration of the peanut extract to a placebo treated patient. The authors indicated that in the three actively treated patients there was a marked reduction of symptom score after food challenge, whereas, the only available placebo treated patient showed no difference in the symptom score after food challenge.
The purpose of this study was to assess if a parenteral immunotherapy program, using a partially characterised crude peanut extract, could induce a state of immunological tolerance in a patient who exhibited anaphylaxis, asthma and urticaria on exposure to peanut and other legumes. A further aim was to measure the serum antibody responses to the immunotherapy.
MATERIALS AND METHODS
Clinical features
A male patient was 14 years of age at the start of this study. He had presented with an initial episode of anaphylaxis at the age of 15 months, following his first known exposure to peanut antigen in the form of peanut butter. As other legumes were introduced into his diet less severe reactions occurred such as urticaria, vomiting and asthma. Adverse reactions were recorded to soybean and derived emulsifiers, peas, beans and lentils. A strict, legume free, diet was prescribed. Despite generally excellent adherence to this diet, the patient required three further hospital admissions for anaphylaxis following accidental exposure to processed foods containing peanut. The most recent anaphylactic episode occurred when the patient was 14 years of age. There was concern over the severity and life-threatening nature of the attacks plus the prospective lifelong limitation in lifestyle resulting from management of the patient's allergy purely on a dietary basis with adrenaline injections. This led to a decision to commence parenteral immunotherapy in an attempt to induce tolerance to peanut and other legumes.
Peanut extract
A partially characterised crude peanut extract (CPE-Raw) was used for the parenteral treatment. This extract was prepared from defatted, ground peanuts as described previously 10. For use, the lyophylised extract was redissolved in physiological saline (1 mg/mL) and sterile filtered through a 0.22 μm pore size Millex-GV membrane (Millipore Corp., Bedford MA) to exclude pathogenic organisms. The potency and specificity of this extract had been investigated in earlier cross radio-immunoelectrophoresis experiments 10 using pooled sera from 10 patients, including that of the subject patient. These studies revealed the presence of at least 16 IgE-binding antigens.
RAST determinations
IgE RAST was performed on serum samples collected before commencement of immunotherapy and throughout the period of treatment. Sera were stored at minus 20 °C. Allergen extracts from peanut, soybean, pea and lentil were made and used in the tests as previously reported 14. IgG4 RAST was carried out retrospectively on the stored serum samples. Peanut allergen discs for the determination of specific IgG4 were supplied by Deakin Research Ltd. (North Sydney, NSW, Australia) and used in accordance with the manufacturer's instructions. The IgG4 RAST method was analogous to the IgE RAST procedure, and was carried out with radio-labelled anti-IgG4 and paper discs specially prepared so as to give low non-specific binding. The 125I-labelled anti-IgG4 was obtained from Pharmacia Inc. (Piscataway NJ).
Immunoblotting
Immunoblotting was carried out using Bio-Rad (Bio-Rad Laboratories, Richmond CA) apparatus 15,16. Extracts were prepared of peanut, soybean, garden pea and lentils as used for RAST. Our method for the electroblotting transfer of allergens followed the recommendations of Baldo and Tovey 17. Sodium dodecylsulfate polyacrylamide electrophoresis (SDS-PAGE) was carried out with the 1 mm gels in the Mini-Protean (Bio-Rad) cell. The polyacrylamide gel contained 12.5 %T monomer. Legume protein samples were made 5 mg/mL in SDS sample buffer. One hundred μL of sample was loaded onto the gel in the slot formed by the blotting template. The voltage was set at 200 V for 45 min for electrophoresis. Electroblotting transfer was made at 100 V for 1 h in Tris/glycine buffer containing 20 % methanol. Transfer in the Transblot cell was made to nitrocellulose membrane (Schleicher & Schuell Inc, Keene NH) of 0.1 μm pore size. Remaining protein-binding sites were blocked by overnight immersion of the membrane in Tris-buffered (pH 7.5, 0.05 M) saline (0.9 %) containing 0.1 % Tween (v/v) and also 1 % BSA and 0.1 % sodium azide (TTBS buffer). Probing of the blots was made following vertical slicing of the nitrocellulose membranes into 5 mm wide strips. Strips from each legume transfer were stained for total proteins with Indian ink (0.5 % v/v in saline/Tween). Incubations were carried out in polypropylene tubes at room temperature. The specific-antibody membrane staining technique was derived from that described in the Bio-Rad Immuno-Blot alkaline phosphatase assay kit 18.
For the detection of specific IgE the following steps were performed:
1. Serum was diluted in TTBS 1:10 and 0.5 mL of this solution was added to the strip in the tube. Incubation of the strip was on a rocker overnight.
2. The strip was washed three times with 1 mL of TTBS with agitation.
3. Anti-human IgE (rabbit) (DAKO A094 DAKO Corp, Carpinteria CA) was diluted 1:100 in TTBS and 0.5 mL of this solution was added to the strip in the tube. Incubation of the strip was on a rocker for 3 h.
4. The strip was washed three times with 1 mL of TTBS with agitation.
5. Swine anti-rabbit alkaline phosphatase (DAKO 306) was diluted 1:500 in TTBS and 0.5 mL of this solution was added to the strip in the tube. Incubation of the strip was on a rocker for 3 h.
6. The strip was washed three times with 1 mL of TTBS with agitation.
7. Color development reagents, 5-bromo-4-chloro-3-indolyl phosphate p-toluidine sale (BCIP) and p-nitro tetrazolium blue (NBT) were made up according to Bio-Rad Corp directions. One mL of the reagents in carbonate buffer was added to each tube and agitated for 15 to 30 min.
8. The strip was washed with distilled water and dried.
For the detection of specific IgG4 the following steps were performed:
1. Serum was diluted in TTBS 1:10 and 0.5 mL of this solution was added to the strip in the tube. Incubation of the strip was on a rocker overnight.
2. The strip was washed three times with 1 mL of TTBS with agitation.
3. Anti-human IgG4 (sheep) (ICN 643131 Miles Scientific, Naperville IL) was diluted 1:200 in TTBS and 0.5 mL of this solution was added to the strip in the tube. Incubation of the strip was on a rocker for 3 h.
4. The strip was washed three times with 1 mL of TTBS with agitation.
5. Donkey anti-sheep alkaline phosphatase conjugate (Sigma-A7789 Sigma Chemical Co., St. Louis MO) was diluted 1:500 in TTBS and 0.5 mL of this solution was added to the strip in the tube. Incubation of the strip was on a rocker for 3 h.
6. The strip was washed three times with 1 mL of TTBS with agitation.
7. Staining reagents BCIP and NBT were made up and used as for the IgE detection.
Sera from two patients with high levels of specific IgE and IgG4 to ryegrass pollen but no apparent legume allergies were used as controls in preliminary probing experiments.
Immunotherapy protocol
Desensitisation commenced with serial dilutions at 10 6, 10 5 and 10 4 of a 1 mg/mL solution of CPE-Raw. Injections were administered at 30 min intervals using a dose of 0.1 mL. Histamine release was observed at the site of injection with the 10-4 dilution. Subsequently, weekly injections were given of the 10-4 dilution with 0.1 mL incremental increases to reach a dose of 0.1 mL of a 10-2 dilution after a period of 9 months. At this time it was found that significant immediate and delayed reactions occurred if the dose (equivalent to 1 μg of CPE-Raw) was exceeded. Therefore the level of 0.1 mL of the 10-2 dilution was maintained for another 17 months, when an increased dose was tolerated for the first time. The dose of antigen was then raised by weekly increments of 0.05 mL over the following two years. Apart from some mild to moderate local skin reaction, there were no apparent ill effects. Thirty-nine months after treatment had begun the patient was tolerating a dose of 0.1 mL of a 1 mg/mL solution of CPE-Raw.
Challenge
The next month the patient was hospitalized for oral challenge. At 20 min intervals he was challenged by ingestion of CPE-Raw, commencing with 10-6 mg and proceeding by 10-fold increments until 0.1 mg of antigen was reached. Excellent tolerance was observed. The regimen was continued and he tolerated further challenges with 0.15, 0.25, 0.5, 0.75 and 1.0 mg at 20 min intervals. Over the following 2 h the patient was challenged with commercial peanut butter. A quantity of peanut butter equivalent to 220 mg of extractable CPE-Raw (880 mg or approximately 1 mL) was ingested and tolerated. The challenge was repeated at 30 min intervals with increasing doses (330,400 and 550 mg of antigen). With the final 550 mg challenge (accumulated dose 1540 mg of antigen) early signs of anaphylactoid reaction were observed and rapidly reversed by administration of epinephrine 1/1000.
Post challenge
After the challenge the patient was maintained on daily peanut butter equivalent to 1500 mg of CPE-Raw antigen with apparently excellent tolerance. Long term maintenance of peanut tolerance is being achieved by an average daily intake of 1.7 g of raw peanut (equivalent to 428 mg of extractable protein). Over the 3 weeks following the hospital challenge with CPE-Raw, the patient was challenged with samples of the legumes (soybean, peas, beans and lentils) previously known to produce reactions and no difficulties were encountered. He now eats these legumes without restriction.
RESULTS
IgE RAST
Initial testing of the patient's serum confirmed the marked clinical sensitivity to both raw and roasted peanut plus to the major peanut protein fractions (table I). Similarly, significant sensitivity to other legumes was corroborated by RAST (table II). Peanut RAST was determined before and throughout the 39 months of immunotherapy (table III and fig. 1). Serum samples were also stored for retrospective examination.
Figure 1.--The IgE and IgG4 responses, recorded as per cent radioactivity uptake, versus the logarithmic expression of the cumulative dose of antigen (CPE-Raw) given over a 54 month period.
IgG4 RAST
The simple disc-based system was not available during the course of the desensitisation program and the values reported were obtained with stored sera and on fresh sera collected after the oral challenge (table III and fig. 1).
Immunoblotting
The results of immunoblots with the 4 legumes are seen in fig. 2. The IgE blots show apparent declines in both intensity and number of bands following immunotherapy. IgG4 results reveal the contrary effect, i.e., increasing intensity and more bands being labeled after therapy. For the allergen blots from peanut, soybean and pea there is close correspondence of band staining between IgE and IgG4.The results for lentil are less clear.
Figure 2.--The results of immunoblotting of SDS-PAGE gels made with the extracts from 4 legumes. The left-hand strip indicates the total protein transferred. Each of the legume extracts gave a characteristic and complex pattern with bands having Mr ranging from approximately 100k (top) to 10k (bottom). The pairs of adjacent strips were exposed to the patient's serum obtained pre- and post-treatment respectively. These pairs were subsequently probed for the presence of specific IgE and IgG4. The figure shows the results from the respective extracts marked as follows: (A) Peanut Arachis hypogaea; (B) Soybean Glycine max.; (C) Garden pea Pisum sativum; (D) Brown lentil Lens culinaris (esculenta).
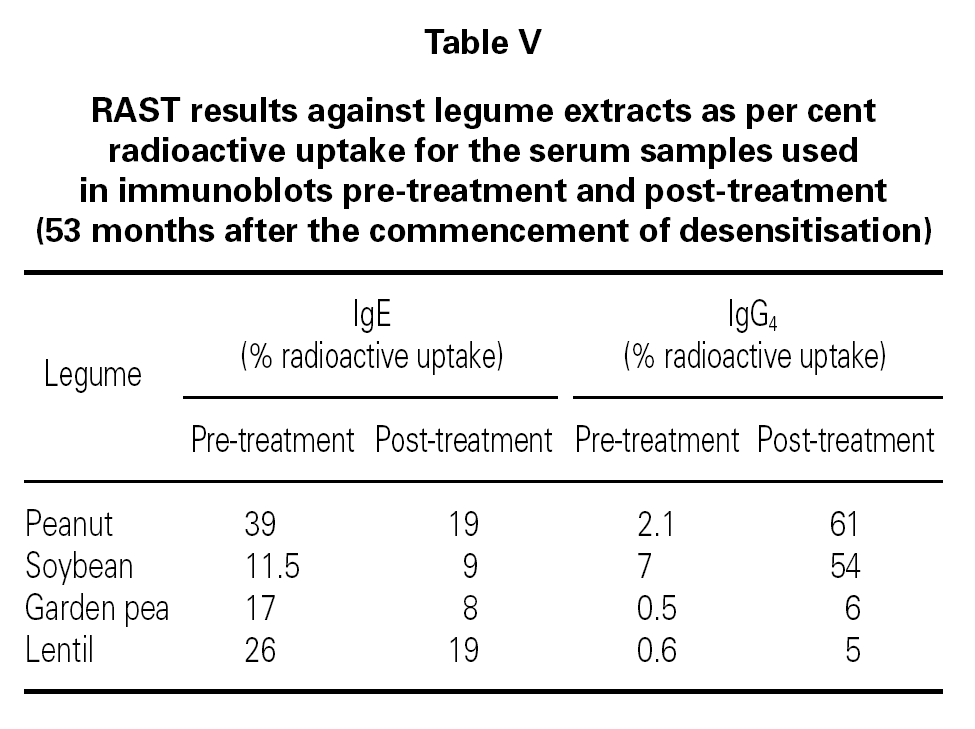
The blots correlate well with the results of IgE and IgG4 RAST recorded in table V. The control sera gave virtually no staining of the legume protein bands and thus are not shown in fig. 2.
DISCUSSION
This study illustrates that parenteral desensitisation to peanut was successful in one patient with severe life-threatening sensitivity. In this patient, sensitivity to other legumes was simultaneously eliminated as shown by tolerance to challenge by ingestion. This implies cross-desensitisation. Potentially, cross-immunotherapy may be of great importance in the treatment of food allergy. We have previously observed extensive cross-reactivity between legumes by RAST in the sera of a group of patients with severe immediate hypersensitivity to peanut 14.
In extremely sensitive patients, such as ours, the interval required to complete a parenteral immunotherapy program can be prolonged. High threshold levels of sensitivity can delay progression for extended periods. However, it is only by achieving tolerance to a high antigen dose that a subsequent oral challenge can be expected to be successful. These factors need to be carefully considered against the motivation and compliance of the patient to attend for weekly injection plus the clinical indications for embarking on such a program. Extreme care needs to be taken in conducting food desensitisation. It should be conducted wholly within a special program by experienced personnel. Most patients with peanut allergy can be successfully managed by dietary avoidance and some may acquire tolerance over time. However, a select group of patients exhibit life-long sensitivity to the point of anaphylaxis on accidental exposure to even minute quantities of peanut. It is this group for which immunotherapy may represent an alternative treatment. The quality and potency of the extract used is an important factor in achieving the desired acquisition of tolerance. The crude peanut extract (CPE-Raw) employed in this study had been shown 10 to incorporate a large number, perhaps all, of the IgE-binding antigens to which the patient was sensitised.
The immunological response in this study followed the pattern observed in bee venom and ragweed pollen immunotherapy. The rise in peanut-specific IgG4 antibody was antigen-dose dependent, being maximised at high dose, while the IgE level fell slowly over time. The 48 month period following challenge was associated with continued tolerance to peanut and other legumes. In our patient this tolerance correlated with his ability to maintain high levels of specific IgG4, which acted as a marker of protection against anaphylaxis. In this paper the technique of immunoblotting allergen-specific IgG4 has been described and has been combined with IgE immunoblotting to illustrate the specific immunologic changes that have occurred as a consequence of the immunotherapy. The matching inverse changes in intensity of bands appear to correlate with the successful outcome of desensitisation. This observation is generally supported by the study of Bonitz, Jarolin, Rumpold et al 19 which used IgE immunoblotting alone in testing the sera of sensitive patients undergoing immunotherapy. The additional use of IgG4 immunoblotting may provide an improved level of discrimination in the assessment of correlation of clinical efficacy with the immunologic response.
Finally, the question remains whether oral desensitization would offer a satisfactory alternative to the parenteral method. We do not have experience with the former but would contend that, at least for the exquisitely sensitive patient, precise control of the antigen dose delivered to the immune system is essential. Only the parenteral method can provide that level of accuracy.
ACKNOWLEDGEMENTS
The authors wish to acknowledge the excellent technical assistance of J. Myers and T. Grigoriadis.