The characteristics of tree nuts (TNs) and peanut (PN) allergies vary in different regions of the world. We aim to identify the characteristics of TNs/PN allergies in Turkish children.
Patients and MethodsA total of 227 children [4.8 (3.2–6.8) years] with TN and/or PN allergies were included. The phenotypical features of TNs/PN allergic children and the risk factors for multiple TNs/PN allergies were evaluated.
ResultsAllergy to TNs/PN developed at a median age of 12.0 (10.0–18.0) months. The most common TNs/PN responsible for food allergies were the hazelnut (63.9%) and the pistachio (54.6%). Of TNs/PN allergic children, 54.2% experienced reactions with at least two types of . Current ages 6–10 years [OR:2.455, 95% CI:1.255-4.852, p=0.009] and family history of atopy [OR:2.156, 95% CI:1.182–3.932, p=0.012] were the risk factors for multiple TNs/PN allergies. Most of the patients with cashew nut and pistachio allergies exhibited co-sensitization and co-allergy to both of these TNs/PN. Although the rarest TNs/PN allergy was seen with almond, the possibility of allergy to other TNs or PN was highly increased in the patients with almond allergy compared to other TNs/PN.
ConclusionsChildren with TNs/PN allergy living in an East Mediterranean region differ from the counterparts living in Western countries by an earlier age of onset of the TNs/PN allergy symptoms, increasing possibility to have multiple TNs/PN allergy at older ages, and different spectrum of TN/PN allergies (hazelnut followed by pistachio/cashew) that all indicate the consumption habits which are important determinants of TN/PN allergy development.
Allergy to tree nuts (TNs) along with peanut (PN) constitutes a significant risk for severe anaphylactic reactions1 leading to limitations in daily life and creating a major burden on health care.2 For these reasons, TNs and PN allergies are distinguished among other food allergies in terms of severity.3
The prevalence of TNs/PN allergies has doubled in recent decades, especially in childhood.4 On the other hand, the prevalence of TNs/PN allergy is extremely variable and highly dependent on cultural consumption habits and agricultural characteristics of societies. In Turkey, many types of TNs/PN are cultivated and consumed in high quantities, but hazelnut accounts for 75% of the world production.5 However, the data on clinical characteristics, prevalence, risk factors of TNs/PN allergies in Turkey is markedly limited, especially in the pediatric age group.6
In this study, we aimed to provide a comprehensive phenotypical characterization of children in the East Mediterranean region with TNs and PN allergy and to reveal the rates of co-sensitization as well as co-allergy between TNs/PN.
Methods and materialsPatientsThis research on TNs and PN allergies in childhood (0–18 years old) was conducted at the Division of Pediatric Allergy in Hacettepe University, which is a tertiary referral center for the country, between January 2010 and March 2018. The study was performed in accordance with the protocol approved by the local ethical committee of Hacettepe University (approval identification number is GO 15/649-07).
Study designTwo hundred and twenty-seven TNs/PN allergic patients who fulfilled at least one of the two following criteria were enrolled in the study.
- 1)
Presence of specific IgE (sIgE) to at least one type of TNs or PN (≥0.35kU/L) or positive skin-prick test (SPT) (≥3mm) along with at least one of the three following criteria:
- a
A consistent (at least two occasions) and clear-cut history of at least one TN and/or PN related symptoms after the ingestion of the causative TNs/PN (n=115).
- b
Positive open food challenge (OFC) tests with causative TNs/PN (n=31).
- c
Anaphylaxis with at least one TN and/or PN within the last 12 months (n=41).
- a
- 2)
sIgE levels and/or SPT wheal diameters of TNs/PN indicating clinical reactivity with >95% accuracy (n=155).
Patients were further analyzed for co-sensitization and co-allergy,14 and grouped as multiple TNs/PN sensitization (SPT was ≥3mm and sIgE value was ≥0.35Ku/L, but these two parameters were below the cut-off values that predict clinical reactivity with >95% accuracy) and multiple TNs/PN allergy (allergy to more than one type of TNs with or without PN).15 Multiple TNs/PN allergy was diagnosed according to the four given criteria above.
All data were collected by the questioning caregivers and reviewing of file records for current age, gender, age of onset of the TNs/PN allergy, initial and subsequent reactions to TNs/PN, family history of atopy, laboratory parameters such as total laboratory parameters including immunoglobulin E (IgE) and sIgE values, eosinophil and basophil counts. The diagnosis of “food allergies other than TNs/PN”, AD, asthma, and AR was met according to international guidelines.16–19 Likewise, reactions during consumption of TNs/PN were defined and categorized based on World Allergy Organization Anaphylaxis Guidelines.20
Study interventionsSkin prick test was done with walnut, hazelnut, almond, cashew nut, pistachio, and peanut (®Stallergenes, SA, Antony, France) for each participant; SPT with pollens (grass, weed, and tree) (®Stallergenes, SA, Antony, France) were applied on upper backs or volar face of the forearms of the children older than two years. For patients younger than two years, SPT with other food allergens (cow’s milk, egg white, soy, wheat, sesame, lentil, chickpea) (®Stallergenes, SA, Antony, France) were performed on the upper backs of the subjects.
Specific IgE for TNs, PN, and total IgE were measured by the Immuno-CAP method in the sera of the patients (Pharmacia & Upjohn, Uppsala, Sweden).
The children whose caregivers gave informed consent underwent OFC with TNs and/or PN based on the recommendations in the guidelines.21 Open OFCs were preferred over double-blind, placebo-controlled food challenges due to simplicity in clinical practice.21 OFC tests were discontinued and accepted positive when objective hypersensitivity symptoms occurred.21
Statistical analysisStatistical analyses were performed using SPSS Version 22.0 statistical software package (IBM SPSS Statistics Chicago, Ill, USA). Categorical values were not normally distributed; thus, the data are given as median and interquartile ranges (IQR). All data including current age, onset age of the symptoms, eosinophil and basophil counts, total IgE, and sIgE values were given as median and interquartile range due to non-normally distribution, and all statistical comparisons were performed using Mann–Whitney U test on ranks as appropriate. The statistical tests were two-sided, and a p-value≤0.05 was regarded as statistically significant.
ResultsA total of 227 children (M/F: 166/61) with a median age of 4.8 (3.2–6.8) years who had TNs and/or PN allergy were enrolled in the study (Table 1). The median age of onset of the diagnosis with TNs and PN was 12 (10.0–18.0) months. The earliest age of onset of the diagnosis occurred with walnut 9.0 (6.0–12.0) and hazelnut 9.0 (6.0–12.0).
Characterization of the study population (n=227).
| Clinical and laboratory parameters | |
|---|---|
| Current age (years)a | 4.8 (3.2–6.8) |
| Age of the onset of the allergic symptoms to TNs (months)a | 12 (10.0–18.0) |
| Age of the onset of the allergic symptoms to PN (months)a | 12 (10.0–18.0) |
| Patients with allergic symptoms following consumption at the time of the diagnosis of TNs/PN allergy (n/%) | 115/50.6% |
| Patients with AD diagnosed as TNs/PN allergy by performing SPT or sIgE with TNs/PN (n/%) | 88/38.8% |
| Patients diagnosed as TNs/PN allergy by performing SPT or sIgE with TNs/PN due to symptoms with foods other than TNs/PN (n/%) | 23/10.1% |
| Gender (M) (n/%) | 161/71% |
| AD (n/%) | 163 (72%) |
| AR (n/%) | 27 (12%) |
| Asthma (n/%) | 82 (36.1%) |
| Accompanying pollen sensitization (n/%) | 33 (14.5%) |
| Anaphylactic reactions after the consumption of any TNs or PN (n/%) | 94 (41.4%) |
| TNs/PN Allergy | |
| Hazelnut (n/%) | 145 (63.9%) |
| Pistachio (n/%) | 124 (54.6%) |
| Cashew nut (n/%) | 96 (42.3%) |
| Walnut (n/%) | 91 (40.1%) |
| Peanut (n/%) | 50 (22.0%) |
| Almond (n/%) | 24 (10.6%) |
| OFC test positivity (n/%) | 242 |
| Hazelnut (n/%) | 6/26 (23%) |
| Pistachio (n/%) | 24/81 (29.6%) |
| Cashew nut (n/%) | 11/50 (22%) |
| Walnut (n/%) | 2/22 (9%) |
| Peanut (n/%) | 2/25 (8%) |
| Almond (n/%) | 1/38 (2.6%) |
| Concomitant Food Allergy other than TNs/PN (n/%) | 141(62.1%) |
| Only Cow’s milk | 17 (17.5%) |
| Only Egg white | 59 (26%) |
| Concomitant cow’s milk and egg white | 55 (24.2%) |
| Wheat | 1 (0.04%) |
| Legumes | 2 (0.08%) |
| Lentil | 2 (0.08%) |
| Sesame/Tahini | 7 (0.3%) |
| Family history of atopy (n/%) | 67 (29.5%) |
| Family history of food allergy (n/%) | 12 (5.3%) |
| Family history of TNs/PN allergy (n/%) | 6 (2.6%) |
| Eosinophil count (/mm3)a | 400 (200-675) |
| Basophil count (/mm3)a | 0 (0-100) |
| Total IgE (kU/L)a | 211 (73.6-473.0) |
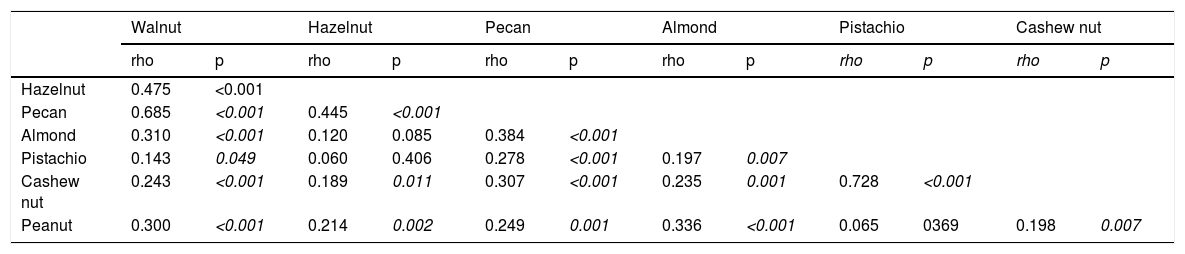
Correlation between SPT wheal diameters of TNs and PN.
| Walnut | Hazelnut | Pecan | Almond | Pistachio | Cashew nut | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| rho | p | rho | p | rho | p | rho | p | rho | p | rho | p | |
| Hazelnut | 0.475 | <0.001 | ||||||||||
| Pecan | 0.685 | <0.001 | 0.445 | <0.001 | ||||||||
| Almond | 0.310 | <0.001 | 0.120 | 0.085 | 0.384 | <0.001 | ||||||
| Pistachio | 0.143 | 0.049 | 0.060 | 0.406 | 0.278 | <0.001 | 0.197 | 0.007 | ||||
| Cashew nut | 0.243 | <0.001 | 0.189 | 0.011 | 0.307 | <0.001 | 0.235 | 0.001 | 0.728 | <0.001 | ||
| Peanut | 0.300 | <0.001 | 0.214 | 0.002 | 0.249 | 0.001 | 0.336 | <0.001 | 0.065 | 0369 | 0.198 | 0.007 |
SPT, Skin Prick Test.
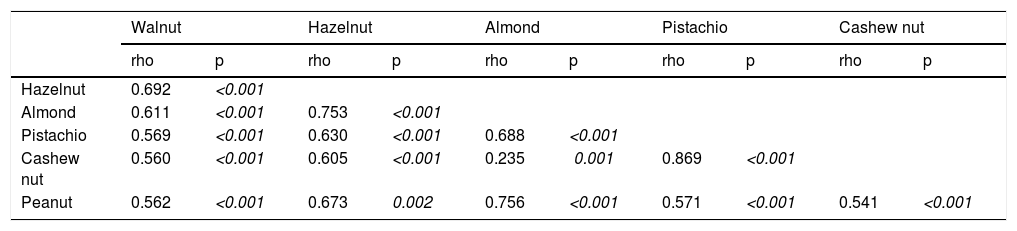
Correlation between sIgE of TNs and PN.
| Walnut | Hazelnut | Almond | Pistachio | Cashew nut | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| rho | p | rho | p | rho | p | rho | p | rho | p | |
| Hazelnut | 0.692 | <0.001 | ||||||||
| Almond | 0.611 | <0.001 | 0.753 | <0.001 | ||||||
| Pistachio | 0.569 | <0.001 | 0.630 | <0.001 | 0.688 | <0.001 | ||||
| Cashew nut | 0.560 | <0.001 | 0.605 | <0.001 | 0.235 | 0.001 | 0.869 | <0.001 | ||
| Peanut | 0.562 | <0.001 | 0.673 | 0.002 | 0.756 | <0.001 | 0.571 | <0.001 | 0.541 | <0.001 |
sIgE: specific Immunoglobulin E.
Patients with concomitant allergic disorders including asthma, AR, AD, and food allergies other than TNs/PN constituted 86% (n=195) of the study group. Among these, AD was the most common allergic disorder (n=163, 72%), and this was followed by concomitant food allergy other than TNs/PN by 62.1% (n=141). (Table 1).
Of 227 children, 94 (41.4%) had anaphylaxis with at least one type of in the previous history or in OFCs. Of the whole study population, 145 (63.9%) had hazelnut, 124 (54.6%) had pistachio, 96 (42.3%) had cashew nut, 91 (40.1%) had walnut, 50 (22%) had PN, and 24 (10.6%) had the almond allergy. In addition, there was no patient in our study group who had ever consumed pecan, probably due to the dietary habits of our country.
Of the study population, five of the patients (2.2%) had only PN allergy, 99 (43.6%) had only one TN allergy, and 123 (54.2%) had more than one TN allergy or at least one TN along with PN allergy. On the other hand, 45 (90%) of the PN allergic patients were also allergic to other TNs.
Two hundred and forty-two open OFC tests with TNs/PN were performed, and the most frequent positivity was observed with pistachio, hazelnut, and cashew nut, and the least positivity was seen with almond.
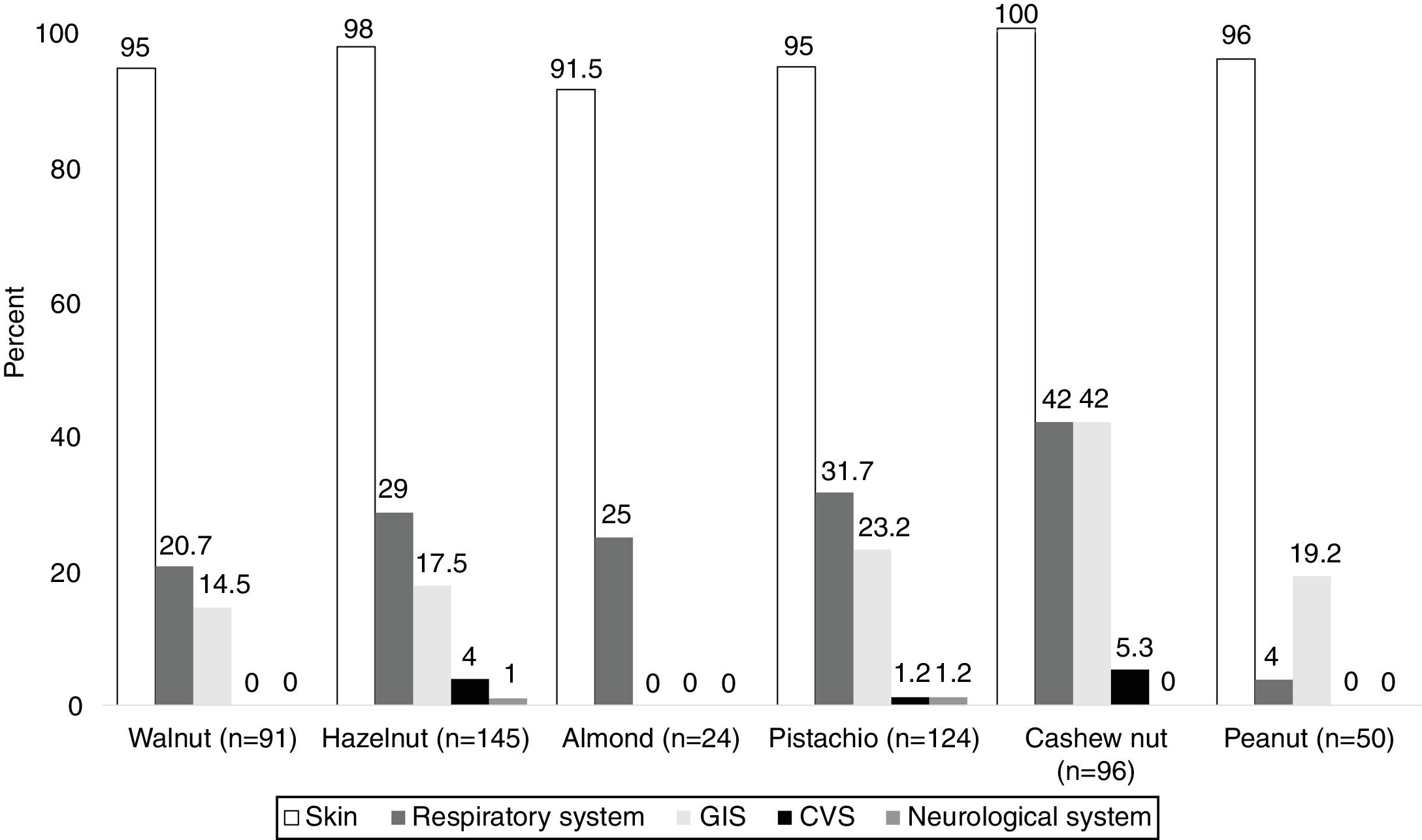
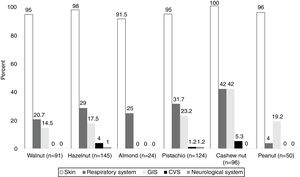
System involvements during hypersensitivity reactions due to TNs and PNThe majority of the subjects (98.5%) presented with cutaneous symptoms, and this was followed by the respiratory (35.5%) and gastrointestinal system (GIS) symptoms (28%). The least commonly occurring symptoms were associated with cardiovascular (CVS) [hypotension (n=2), loss of consciousness (n=1)] (2%) and neurological systems [seizure n=1)] (1%).
In PN allergies, GIS was the second most commonly involved system during hypersensitivity reaction. In addition, respiratory system and GIS involvement were most frequently observed in patients with the cashew nut allergy (42%) (Fig. 1).
Single vs. multiple TNs/PN sensitizations and allergyOverall, 164 patients (72.2%) based on SPT and 142 patients (62.6%) upon sIgE results were sensitized to at least two types of TNs with or without PN (multiple TNs/PN sensitization). Nevertheless, only 123 subjects (54.2%) had multiple TNs/PN allergy. While the proportion of multiple TNs/PN allergy continuously increased by age (36.5% at two years old, 60.5% at six years old, 83.3% at 10 years old, 50% at 14 years old) up to 10 years, then decreased and stayed on the same level throughout adolescence.
In addition, current ages 6–10 years [OR:2.455, 95% CI:1.255-4.852, p=0.009] and family history of atopy [OR: 2.156, 95% CI: 1.182–3.932, p=0.012] were the risk factors for multiple TNs/PN allergies determined in multivariate analysis.
Co-sensitization between SPT and sIgE measurements of TNs and PN, and co-allergy between TNs/PNThe strongest co-sensitization correlation coefficient with respect to SPT wheal diameters existed between pistachio and cashew nut (r=0.715, p<0.001) (Table 2a). While the weakest correlation was observed with TNs, the strongest correlation was seen with almond in PN allergic children (r=0.456, p<0.001). In addition, with regard to sIgE results, the strongest co-sensitization correlation coefficient was observed between pistachio and cashew nut (r=0.869, p<0.001) (Table 2b).
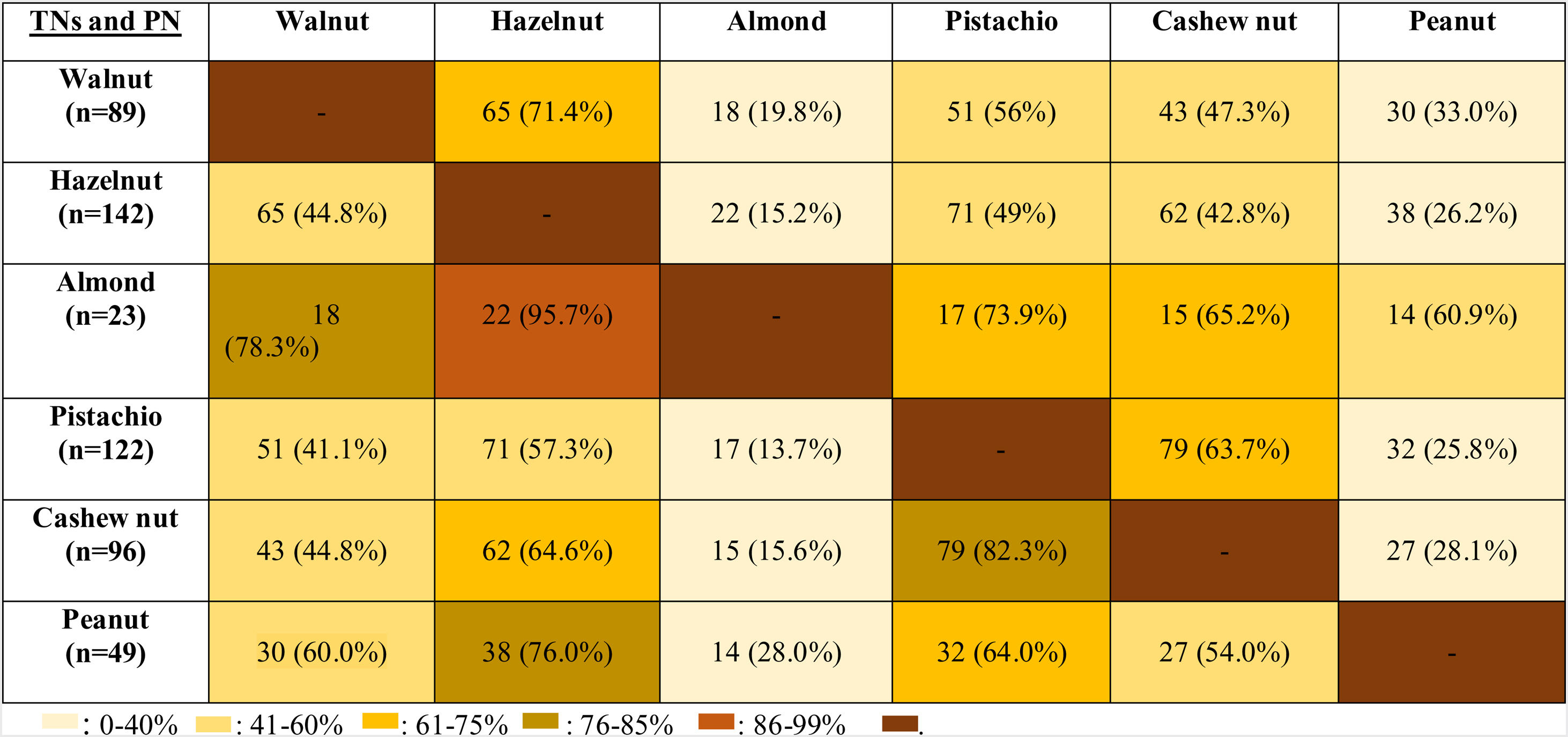
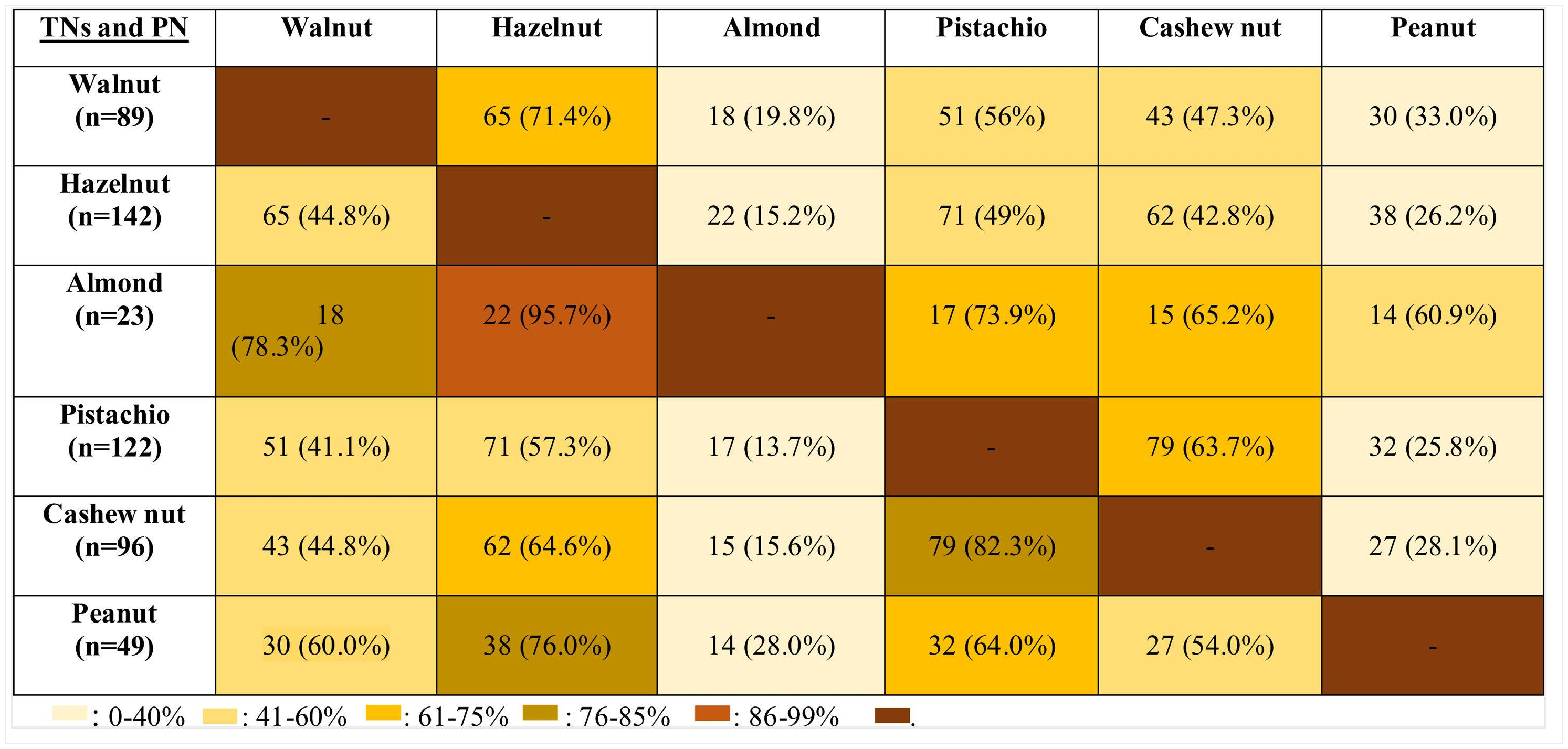
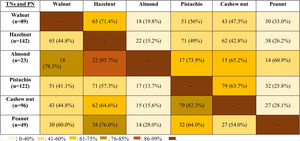
The co-allergy relationship between all TNs and PN was analyzed (Table 3), and patients with the almond allergy had the highest frequency of co-allergy with all other TNs/PN. This was followed by the co-existing allergy with pistachio in cashew nut allergic children (82.3%). Moreover, PN and walnut allergic children were co-allergic to hazelnut in relatively higher ratios (76% for PN, 71.4% for walnut).
DiscussionThis study emphasized earlier onset age of the hypersensitivity symptoms with TNs/PN and higher frequency of allergy with hazelnut, pistachio, and cashew nut in Turkish children. The prevalence of multiple TNs/PN allergy increased up to the age of 10 years after which it decreased and then reached a steady level. The strongest co-sensitization and the most frequent co-allergy were determined between pistachio and cashew TNs/PN allergic children. Although almond allergy was the rarest experienced TN allergy in our study population, the most frequent co-allergy was observed in almond allergic children to all other types of TNs/PN.
One of the most important outcomes of the current study is the earlier age of onset of the TNs/PN related symptoms in Turkish children. We showed that children experienced the first reactions with TNs/PN at the age of one. In a study,15 TNs/PN related allergic symptoms have been reported by the age of two. Moreover, Sicherer et al. reported that the median age of onset of the reaction with TNs was about 36 months, whereas the age of onset of allergy with PN was 14 months.22 Earlier age of onset of the TNs/PN related symptoms in allergic Turkish children may be the effect of early cutaneous exposure to TNs/PN allergens within household dusts before introduction to complementary foods due to the high consumption of TNs/PN for cultural reasons. Moreover, the age of onset of allergic symptoms with walnut and hazelnut was earlier than the symptoms of other TNs/PN in our study. The underlying reason of an earlier age of onset of the symptoms with those two TNs may be the relatively high cultivation as well as consumption habits of walnut and hazelnut in Turkey.
In the current study, allergy to hazelnut, pistachio, and cashew nut was the most commonly observed TNs/PN allergy in children. In the literature, characteristics of allergy to the TNs/PN species are extremely variable.3,23,24 The most common TN allergies are walnut3 and cashew nut3,23 in the United States, but hazelnut and walnut in Spain.25 Remarkably, the presence of pistachio allergy in Turkey has been emphasized, and its prevalence has been reported as 6.7% in food allergic children.6 The possible reason for this higher frequency of pistachio and hazelnut allergies in the current study is that pistachio and hazelnut are grown, exported, and consumed in relatively higher amounts in Turkey compared to other countries.5 In addition, cashew nut shows a higher frequency of co-sensitization and co-allergy with pistachio than the other TNs. Therefore, the wide variety in the allergy prevalence and characteristics of TNs depends on the geographical region and cultural eating habits.26
Similar to previous reports,27 skin (98%) was the most frequently involved system upon TNs/PN consumption, and this was followed by respiratory and GIS system in the current study. Likewise, symptoms of respiratory system and GIS have been frequently reported in TNs/PN allergic children following skin involvement.28,29 In a recent study,30 the respiratory system has been associated with severe anaphylaxis in TNs, but not in PN. Likewise, allergic reactions with TNs, especially with cashew nut, were more severe and related to respiratory system more frequently than with PN in our study. Furthermore, we observed that CVS and neurological symptoms were more common with cashew nut, pistachio, and hazelnut. Similarly, CVS symptoms have been prominently reported in cashew nut allergic patients in the literature.28,31 Further precautions should be taken while performing OFC with TNs, especially with cashew nut in clinics. Also, clinicians should make an anaphylaxis emergency plan for patients with TNs/PN allergies, including the prescription of adrenaline auto-injector.
Children with TNs/PN allergy can have co-sensitization and co-allergy to other types of TNs/PN.15 The increase of prevalence of both multiple TNs/PN allergy and sensitization by age has been previously reported.15 As distinct from that study,15 multiple TNs/PN allergy increased up to 10 years, then decreased and remained at a stable level after that age in our study. Although the possible reason for the different age pattern of multiple TNs/PN allergy is not clear, this may be due to cultural influences on consumption habits of TNs/PN, development of tolerance by age in a subgroup of those patients, and an increase in the prevalence of TNs/PN allergies in younger generations.
The strongest correlation upon both SPT and sIgE results occurred between pistachio and cashew nut because both pistachio and cashew nut belong to the Anacardiaceae botanical family.32 We showed that PN had the strongest correlation with almond. To share common IgE-binding epitopes of major peanut allergen Ara h 2 with almond33 is due in part to this relationship.
Although allergy to almond was the least observed TNs allergy in our study population, the most frequent co-allergy occurred in almond allergic children to all other TNs/PN. Therefore, we thought that the occurrence of almond allergy was rare, but the presence of almond allergy might be the sign of the high burden of TNs/PN allergy. In the current study, since almond allergy was the least frequently encountered TNs allergy, beginning with almond to the diet of TNs/PN allergic children may be a safe alternative of TNs. The second most common co-allergy was seen in cashew nut allergic subjects to pistachio (82.3%) and walnut allergic children to hazelnut (71.4%). In one study, all pistachio allergic patients were shown to be allergic to cashew nut.34 In contrast, we identified only 63.7% of pistachio allergic patients as co-allergic to cashew nut. Van der Valk et al.35 demonstrated that only nine of 20 patients (45%) with pistachio allergy exhibited co-allergy to cashew nut similar to our study. Interestingly, our results showed that PN allergic children had 76% co-allergy to hazelnut, but hazelnut allergic patients had only 26.2% co-allergy to PN. Similarly, Masthoff et al. reported the high ratios of co-allergy in PN allergic patients to hazelnut, whereas those with hazelnut allergic patients have a lower ratio of PN allergy by 48%.36 However, the underlying mechanism of this multifaceted relation should be clarified.
Allergy to almond is rarely seen among all TNs allergies34; however, almond allergy was found to be 10.6% in the current study. In the USA, almond allergy has been reported from 9% to 15% among TNs allergies,22,37 whereas the prevalence ranged from 22% to 33% in the UK.3 The study by Luyt et al. demonstrated almond sensitization (61.9%) and allergy (7.4%) in higher ratios in South Asian children,38 as shown in the current study. This higher ratio of almond allergy in South Asian children was clarified by them being more likely to have household almond exposure during daily dietary habits.38 Our results confirm the literature data that prevalence of TN allergy highly differs according to agricultural status, dietary routine of the societies, and therefore household contact of children with TNs/PN.
The limitation of this study is that the diagnosis of food allergy was not totally based on DBPCFCs, which is the gold standard for the diagnosis of food allergy. However, the presence of a clear-cut history or positive open OFC, together with positive TNs/PN sIgE and/or SPT, or to have sIgE levels and/or SPT wheal diameters indicating clinical reactivity with >95% accuracy minimize the uncertainty of the diagnosis of TNs/PN allergy. sIgE levels of TNs/PN in Turkish children13 have been found to be lower than children with TNs/PN allergy living in Western countries. However, we accepted Western population data for TNs sIgE cut-off values, which are higher than the levels of our patients, to predict clinical reactivity with >95% accuracy. The most important considerable strength of the current study is to be the first study on the TNs/PN allergy spectrum from the East Mediterranean region. Moreover, a comprehensive overview of cross-reactivity and co-sensitization among TNs/PN has been revealed.
In conclusion, TNs/PN allergic children living in the East Mediterranean region have similar characteristics in terms of severity, co-sensitization, concomitant allergies, and co-allergies compared to counterparts living in Western countries. However, earlier age of onset of the symptoms, clinical spectrum, and age pattern of multiple TNs/PN allergy exhibited variabilities, and all these dissimilarities support the role of cultural dietary habits of the societies in the development of TNs/PN allergies. In addition, although almond may be a good choice for the consumption of children with TNs allergy, allergy to almond may be a sign of multiple TNs/PN allergies.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
All authors approve the final version of the manuscript
All parents/guardians provided written informed consent
The study was performed in accordance with the protocol approved by the local ethical committee of Hacettepe University (approval identification number is GO 15/649-07)
Conflict of interestThe authors have no conflict of interest to declare.