Eosinophilic oesophagitis (EO) is a chronic inflammatory disease of the oesophagus, with an emergent character, defined by the presence of a dense infiltrate by eosinophilic leukocytes restricted to the mucosa of this organ after excluding gastro-oesophageal acid reflux. It is manifested by chronic and/or recurrent dysphagia and episodes of oesophageal alimentary impaction, with great variation in terms of intensity, frequency, and duration of the attacks.
MethodsAn Internet-based search was performed for the most recent articles with relevant information concerning immunopathological mechanisms involved in EO.
ResultsBibliographical data allow us to define that EO is related to an allergic or hypersensitivity-induced reaction after exposure to foods or inhalants, with increased prevalence of sensitisation to these allergens. Data published up to now suggest a cellular hypersensitivity reaction rather than a humoral one in the physiopathology of EO. In this disease, sensitised T-lymphocytes mediate a Th2 type response, releasing cytokines such as IL-5, with a possible Th1 component that requires further investigation. The function of the abundant CD8+ T-lymphocytes present in the oesophageal epithelium has yet to be explained. Mast cells also participate in epithelial inflammatory infiltrate in EO, and it is still unknown if its activation, mainly through IgE, contributes to the immunopathology of the disease even though EO rarely manifests immediate hypersensitivity reactions. IL-5 and different forms of eotaxins perform an important active role in the recruitment of eosinophils to the oesophagus.
ConclusionsEO is an immunologically complex and little studied entity that is associated with other allergic diseases and in which different effector cells participate, determining an immunological response of cellular rather than a humoral hypersensitivity reaction. The data available point out that EO is a disorder of the Th2 retarded immune response, in which the triggering factor might not be IgE. Although the final inflammatory phenomena observed in EO are common for the different patients, the cascade of inflammatory mediators that lead to them might not be identical in all cases, and the morphological and functional disorders observed in EO would represent the final convergence of different activation forms of the mechanisms of inflammation.
The mucosa of the digestive tract is a large surface with connection with the external medium, which has a primary function as a physiological barrier, but, at the same time, it has to possess mechanisms to identify substances and microorganisms that are in contact with it, and with respect to which they will act differentially as tolerant or reactive. The digestive tract mucosa is equipped with several defence mechanisms, both innate and acquired, that protect the individual from the pathogenic action of microorganisms or eliminate transformed cells; at the same time it allows the absorption of partially digested dietary components being tolerant to them. Several functional and structural specialisations on the wall of the digestive tube exist with this purpose, and cells of different lineages with immunological functions live within it, some with a diffused location and many of them grouped in the form of follicles or lymphoid aggregates, which guarantee these functions.
The oesophageal epithelium possesses a different histological structure than the remaining organs of the digestive tract; its flat epithelial cells are situated in different layers, lack secretory or absorption functions, and offer the aspect of a mere lining of a duct. Although acinar glands, lubricating mucous and bicarbonate secretors exist in the submucosal layer these cells are very scarce in comparison with other sections of the digestive tract. In addition, the presence of resident cells from innate immunity or lymphoid aggregates is negligible in comparison with other more distal sections, which are characterised by the presence of companion bacteria and for possessing absorption functions. The structure of the oesophageal mucosa is constituted like a passage duct, although, like every epithelial surface, it possesses its own surveillance system. The oesophageal infiltration by eosinophils reflects the immune response capacity of the organ and indicates the type of effector cell that could be responsible for the inflammatory profile.
Eosinophilic oesophagitis (EO) is a recently described clinical-pathological entity1. The number of diagnosed and reported cases originating from several developed countries has increased exponentially in the last few years, causing it to be considered an emerging disease2,3. It consists of a chronic inflammatory disorder restricted to the oesophageal mucosa manifested with oesophagus-related symptoms, such as recurrent dysphagia and frequent episodes of food impactation, with great variability in terms of intensity, frequency and duration of the attacks4. It should be considered in the differential diagnosis of dysphagia, especially in young males who present other allergic displays. In EO, dysphagia seems to be a consequence of an inflammatory response and not an anatomical obstruction of the oesophagus to food passage5. Also called allergic oesophagitis6 it is associated with other atopical manifestations in a very high number of cases, and it is related with exposure to alimentary or environmental allergens7. The recognition of EO makes it necessary to expand the standard motor function of the oesophagus as a passage duct from the pharynx to the stomach, to include its immunological functions8.
Different works, a great majority in the last 5years, have shed light on different clinical, physiopathological, and therapeutic aspects of EO, but until now we lack a conclusive hypothesis that completely explains the genesis and mechanisms that lie beneath this disease. The present article supposes a revision of the most recent discoveries in the immunopathology of EO, analysed from the cellular and molecular bases of the inflammation and gastrointestinal allergy.
DIGESTIVE MUCOSA AND GALT: IMMUNOLOGICAL TOLERANCE AND GASTROINTESTINAL ALLERGYThe intestinal lymphoid tissue is formed by resident lymphocytes located in Peyer's patches, in the lamina propria and with an intraepithelial localisation. The lymphocyte population of the digestive mucosa is anatomically, phenotypically and functionally compartmentalised in inductive sites (Peyer's patches and mesenteric lymph nodes) and effector sites (lamina propria and intraepithelial compartments)9. Peyer's patches are located throughout the small intestine, being more abundant and developed in the distal ileum. The oral mucosa and the colon (specially the appendix) and rectum are places for the controlled capture of antigens and activation of non-stimulated B and T-lymphocytes. In addition, the mucous lymphocytes stimulated in one region can travel to other mucous surfaces and construct the so-called Mucosa-Associated Lymphoid Tissue (MALT) in which the immunisation at any level (nasal, oral, intestinal, bronchial) can induce protective responses in all the mucous surfaces. Other immune cells which reside in the mucosa are: Langerhans cells, mast cells and dendritic cells. As opposed to lymphoid tissue in other peripheral locations, resident eosinophils can be found forming part of it. Although plasma cells that produce immunoglobulin (Ig) G and IgE are located mainly in the bone marrow and its products circulate in the blood, the majority of IgA secreting cells in the human body are located in the Gut-Associated Lymphoid Tissues (GALT) and the respiratory mucosa. This IgA has the capacity to form complexes with antigens in the intestinal lumen without activating the complement system, preventing its penetration in the organism. Other cells that participate in the immune function of the gastrointestinal mucosa are the regulating T lymphocytes (which generate tolerance) that maintain controlled local responses versus commensal bacteria or dietary components, avoiding an inflammatory response against these innocuous antigens10,11.
The loss of the capacity of immunological tolerance of the digestive mucosa against different intestinal lumen antigens triggers immune responses that result in pathologic inflammatory reactions that affect the wall of the digestive tract. In the past few years the incidence of food allergy has significantly increased, a result probably related to the social-economical development in industrialised countries like the "hygiene hypothesis" proposes12. At the same time, several scientific works have increased our knowledge about the pathological characteristics of food hypersensitivity.
Taking into account its physiopathological mechanisms, food allergies can be classified into three large groups, depending on whether the allergic reaction is completely mediated, partially mediated, or independent of the participation of IgE13,14. On the other hand, food allergies in both children and adults can affect different sections of the gastrointestinal tract. All of this allows food allergy to present itself as different clinical entities, such as the oral allergy syndrome; food protein-induced enterocolitis; allergic constipation; and other forms of dysmotility (this could include some cases of irritable bowel syndrome); and gastrointestinal diseases related to eosinophils13,15.
Allergic gastrointestinal diseases mediated by eosinophils constitute a small group of recently emergent pathologies that are the object of increasing interest by the scientific community16. Although the physiopathological mechanisms for these diseases have not been elucidated at all, more and more clinical and experimental evidence is becoming available which is leading different authors to propose mixed mechanisms mediated and non-mediated by IgE in the genesis of these diseases14,17.
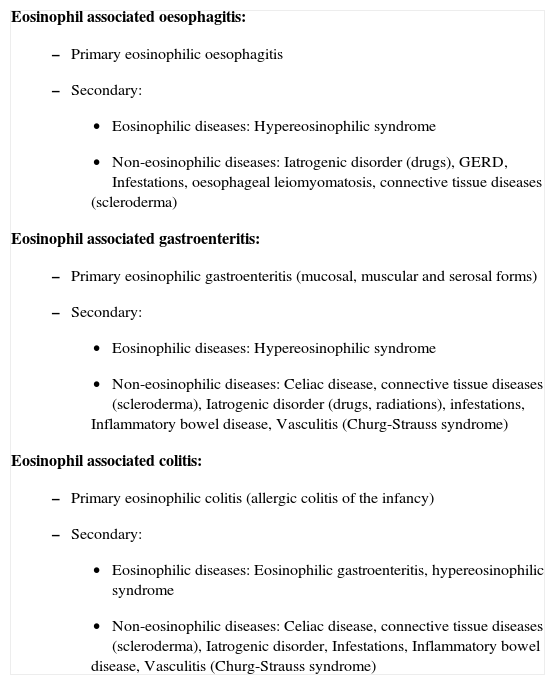
EOSINOPHILS AND DIGESTIVE DISEASESIn many gastrointestinal disorders, an increased number of eosinophils can be detected (Table I). In some of them, like eosinophilic gastroenteritis, eosinophils act as the main effector cell in damaging the tissues18. In others, like inflammatory bowel disease and gastro-oesophageal reflux, the presence of eosinophils possesses an uncertain physiopathological significance, since in these diseases, eosinophils can range from not being present to actively secreting the contents of their granules19–24; it is thought that in these cases, eosinophils could be performing a regulatory function25. Their presence in the oesophagus is observed in different pathological processes, like gastro-oesophageal reflux disease (GERD)26 (for many years the presence of these cells and the diagnosis of the disease were considered synonymous26,27), after caustication of the organ, infections, parasitisations, pharmacological reactions, haematological neoplasia, Crohn's disease, radiations, etc.
Classification of gastrointestinal diseases associated to eosinophils
Eosinophil associated oesophagitis:
|
Modified from Rothenberg ME, 2004.
Allergy, genes and GERD, constitute a combination of possible causes of EO: it is an atopy-associated inflammatory disease, the cause of which is poorly understood. Allergy has been involved because the majority of patients have evidence of personal or familiar history of asthma, allergic rhinitis, atopic dermatitis, hypersensitivity to drugs, foods or aeroallergens, blood eosinophilia or elevated levels of seric IgE28,29. Positive skin prick responses and RAST results have usually been described in patients with EO. Furthermore, the resolution of histological lesions in patients submitted to elemental diets (lacking allergic capacity) has also been reported30. A satisfactory clinical response to antieosinophilic therapies is also observed in EO, thus it is evident that eosinophilic leukocytes play an important role in the development of dysphagia. In addition to this allergic hypothesis, some authors have suggested that gastro-oesophageal reflux (GER) could play any etiological role in EO, by inducing abnormal immunological responses31
Immunological mechanisms of gastrointestinal diseases associated to eosinophils in general, and particularly in EO, have been investigated through different strategies32:
- a)
Immunohistochemical staining of oesophageal mucosa to define the nature of the inflammatory infiltrate of EO.
- b)
Study of the expression of cytokines in populations of blood-circulating lymphocytes obtained from cases of oesophageal eosinophilic infiltration36.
- c)
Analysis of the capacity of T lymphocytes extracted from patients with EO and cultivated under determined conditions for the production of cytokines of a specific inflammatory profile37,38.
- d)
Analysis of gene expression of proinflammatory cytokines in samples of normal mucosa, and also from patients with EO39–41.
- e)
Development of experimental animal models of eosinophil infiltration of different segments of the digestive tract, trying to reproduce the conditions that will trigger, perpetuate, or regulate the infiltration by eosinophil leukocytes37,42,43.
The immunohistochemical staining techniques implemented to samples of oesophageal mucosa and other sections of the digestive tract have allowed us to identify distinct cellular types and learn a great deal of data on the nature of the inflammation and on the immunological capacity of the oesophagus. Nowadays, we know that under normal conditions human oesophageal epithelium possesses all the cellular types needed for the development of a local immunological response44, these are: antigen-processing and presenting Langerhans cells, T-lymphocytes and effector cells (like eosinophils, mast cells, and maybe plasma cells), whose densities noticeably increase in EO with respect to GERD and in healthy control individuals45,46. The number of these inflammatory cells in the gastric and duodenal mucosa does not differ between patients with EO and healthy subjects, which suggests that the inflammatory process in this disease is restricted to the oesophagus7,47.
T-LYMPHOCYTES IN EOSINOPHILIC OESOPHAGITISThe biology of eosinophils is closely regulated by T-lymphocytes by means of different molecules: the development of blood eosinophilia and the eosinophilic infiltration in the lung in response to allergens is integrally dependent of this T cells. Eosinophils of athymic mice (almost completely lacking T-lymphocytes) respond in an altered way to allergens and parasites, and present an anomalous behaviour in situations of inflammation23. T-cells also seem to perform a central function in EO43,48. The normal human oesophageal epithelium contains T-lymphocytes; a decade ago, it was established that their numbers increased in the presence of GER49. More recently, different works have described that in EO the number and density of these cells increases dramatically with respect to healthy control groups and patients with GERD33,46,50, in both pediatric33,51 and adult cases46. The CD8+ T-lymphocytes prove to be predominant in EO, since they make up to three quarters of the total T-lymphocytes, in the same way that occurs in other sections of the digestive tract52.
CD4+ T-lymphocytes and Th2-type responsesMurine experimental models of EO have defined that this disease responds to an immune cellular type reaction42,43. In the generation of which responses mediated by T-cells with a Th2-type cytokine secretion profile have been implicated, with a central role for interleukin (IL)-5 in the induction of eosinophilic inflammation39,47,53,54.
Th2-type responses are mainly mediated by T helper CD4+ cells, which are mainly producers of Th2 type cytokines such as IL-4, IL-5, IL-9, and IL-13. Th2 lymphocytes are powerful activators of the production of antibodies by B-cells, especially of IgE, essentially through stimulation by IL-4 and IL-1355, but they can also induce the recruitment and activation of effector cells like the eosinophil through the production of IL-5 and diverse chemokines.
The majority of studies about eosinophilic tissue recruitment have taken place in the lung and have shown an integral role for IL-556–58 as an important factor for the proliferation, differentiation, survival, and activation, not only of eosinophils, but also of helper T lymphocytes and mast cells in chronic allergic reactions. IL-5 has been clearly implicated in the physiopathology of allergic asthma57,58 and in fibrous remodeling phenomena that occur in bronchial59 and cutaneous60 inflammation. In the investigation of the role of this cytokine in EO, transgenic mice, models were developed that over-expressed IL-5 which caused them to experience an increase in eosinophils circulating in blood and an intense accumulation of eosinophils in the oesophageal lamina propria and the small intestine, proportional to the seric concentration of IL-57,22 when stimulated with allergens by inhalatory 42,61,62 or epicutaneous way63. In the opposite case, mice with a deficiency of this cytokine did not develop eosinophilic oesophagitis when exposed to aeroallergens42. These findings strongly suggest the existence of a type Th2 immunological response in the pathogenesis of EO37. Although experience in humans is limited, we also have evidence that patients with EO show elevated levels of Th2 cytokines, like IL-4, IL-5, and IL-1337,40,64 in their oesophagi. The production of IL-5 by blood lymphocytes in patients with EO also shows a significant increase in comparison to normal controls65, this occurrence can also be observed after its stimulation in vitro38. Furthermore, the percentage of blood circulating IL-5+ CD4+ T-cells correlates with the degree of oesophageal tissue eosinophilia65.
The importance of the effect of IL-5 has been made evident in recent clinical trials which demonstrate a significant reduction in the eosinophilic oesophageal infiltrate and in blood eosinophilia after treatment with a monoclonal humanised anti-IL-5 antibody (MepolizumAb) in short series of patients with EO66 and hypereosinophilic syndrome67. On the other hand, a study that analysed changes induced by treatment with topical steroids on the expression of eosinophilotropic cytokines in a series of eight patients with EO that had normalised oesophageal histology showed that in some cases, the remedying of the inflammatory epithelial infiltration was not associated to changes in the gene expression of IL-541. These results suggest that the effect of this cytokine in itself might not be enough to explain the molecular pathology of EO, forcing us to consider the synergic effect of other molecules, such as, eotaxins, IL-3 and GM-CSF, which have also been directly implicated in the proliferation and accumulation of eosinophils in response to allergens, in its post-mitotic regulation, survival, activation, and capacity of response to other signals7,23. Mast cells and activated eosinophils are capable of secreting these cytokines, which could mean that an autocrine process could exist that, at least partially, would be responsible for the survival and accumulation of eosinophils in tissues. Currently no study to determine the gene expression of IL-3 and GM-CSF in EO exists, but we can turn to some experience obtained from animal models that would support this event61,62.
The potential role of eotaxins in the physiopathology of EO has recently been analysed: eotaxins are a subfamily of chemokines (which groups 3 molecules named eotaxin-1, 2, and 3) which act like potent chemoattractors specific for eosinophils through chemokine receptor (CCR)-3, which are primarily found in these leucocytes68. Eotaxin-1/CCL11 has been the most studied chemokine in the digestive tract69–71, where it is found ubiquitously expressed and its mRNA can be isolated from the mononuclear resident cells in the lamina propria of the small intestine, which is the zone where most gastrointestinal eosinophils reside in normal conditions. Studies on mice deficient in eotaxin-1/CCL11 show that these mice possess less eosinophils in all of the segments of the digestive tube, even when stimulated by allergens and in the presence of elevated levels of IL-5, because the absence of eotaxin-1 blocks the recruitment of eosinophils in the gastrointestinal tract and the lungs, thus increasing the number of blood eosinophils42. Therefore, eotaxin-1 is critical for the recruitment of eosinophils in the gastrointestinal tract through a tissue-specific effect72. Elevated levels of eotaxin-1 are associated to different inflammatory diseases of the human respiratory tract and correlate with the clinical gravity of the process73–75. Although an increase of serum levels of eotaxin-1/ CCL11 in EO has not been observed76, this chemokine seems to perform an important role in both murine42 and human41 EO.
Other studies have centred their interest on eotaxins-2/CCL2439 and more recently on eotaxin-3/ CCL2640, after observing that the latter was the most intensely overexpressed in epithelial cells of the oesophagus of patients with EO with respect to control subjects: the mRNA levels of eotaxin-3/CCL26 and its protein established a very strong mutual relation with eosinophilia and tissular mastocytosis, at the same time that its plasmatic levels were higher than the control subjects. Single nucleotide polymorphism (+2496T > G, rs2302009) in the eotaxin-3 gene is associated with susceptibility to suffer EO40, and mice deficient in eotaxin CCR-3 receptor gene are protected against developing experimental EO. The proportion of peripheral blood eosinophils and its CCR-3 expression are elevated in patients with active EO, in comparison with non-atopic control, and establish a positive correlation with the degree of oesophageal eosinophilia and the tissular expression of the eotaxin-3 mRNA in the oesophageal epithelium65.
However, the potency of eotaxin-1/CCL11 as a ligand of CCR-3 appears to be at least 10 times greater than that of eotaxin-3/CCL2677, therefore modest changes in the gene expression of eotaxin-1 could also perform a relevant function in the recruitment of eosinophils towards the oesophagus41.
RANTES (Regulated upon Activation, Normal T-cells Expressed and Secreted chemokine) is another chemokine involved in inflammatory processes, whose genetic expression appears slightly increased in murine EO with respect to control epithelia69,71 and also in a series of children with EO compare with healthy subjects39. Both RANTES and eotaxins are produced exclusively by inflammatory cells, since they are not detected in epithelial cells of the oesophagus, although it has been recently shown that IL-4 and IL-13 are capable of selectively inducing the gene expression of eotaxin-3 in cutaneous keratinocytes78. Something similar occurs in the case of the epithelial cells of the oesophagus, since a recent work has demonstrated that, after treatment of primary oesophageal epithelial cultured cells with IL-13, a global expression transcript profile that remarkably overlapped with the EO-specific oesophageal transcriptome was observed79. Furthermore, oesophageal epithelial cells markedly produced eotaxin-3 after IL-13 stimulation, and increased IL-13 mRNA levels and the EO transcripsome were largely reversible after glucocorticoid treatment in vivo.
T CD8+ limphocytesThe Th2 responses described to date in the physiopathology of EO are fundamentally mediated by CD4+ helper T-lymphocytes, but the lymphocytary infiltrate in EO is predominantly CD8+33,46. Different studies support evidence for the contribution of T cytotoxic (Tc) cells in the pathogenesis of allergic diseases. Tc cells have been divided into two subsets that secrete Th1 or Th2 cytokines (Tc1 or Tc2). The contribution from Th1 profile cytokines (those of which IFNγ and TNFα are clear exponents) to the physiopathology of the disease is controversial. Conceptually, Th1 cytokines could act like counter-regulators of Th2 reactions, but the concurrent expression of Th1 and Th2 interleukins exacerbates the symptoms, mainly in chronic processes80. This suggests that once a Th2 cell response has been established, Th1 counter-regulation is more complex80. Straumann et al. found an increased expression of TNFα in oesophageal biopsies from 8 adult patients sufferers of EO37, and Gupta et al. have reported an over expression of IFNγ gene in oesophageal epithelium in a series of children affected by this disease39; for this reason we should consider that the inflammatory cascade mediated by Th1 could also play some type of function in the pathogenesis of EO, at least at the local level, since the production capacity of TNFα by blood lymphocytes from patients with EO did not increase with respect to control subjects38,47.
The main function of CD8+ T lymphocytes is the MHC class I-restricted cytotoxicity (Tc1), a function that has yet to be researched in EO. Nevertheless, we should consider the possibility that CD8+ T lymphocytes, maintaining their phenotype, could show a reduced Tc1 function, and on the other hand, could act like cytotoxic-2 (Tc2) T cells. This functional change would be a result of the presence of IL-4 (which could proceed from CD4+ Th2 T lymphocytes from the epithelial infiltrate), which induces the Tc2 lymphocyte to produce more IL-4 and IL-5 with a fundamental function in the recruitment of eosinophils19,81,82 and support to B-lymphocytes for the production of IgE81–83; as a result, CD8+ T lymphocytes would act as an enhancer of tissular inflammation in allergic diseases.
NK lymphocytesThrough flow cytometry NK cells (classically considered potent IFNγ secretors) that expressed IL-5 have been identified in circulating blood of patients with EO, in a significantly greater number than in non-atopic controls65. We do not know the concrete pathological meaning of this event, although recent studies have described a subtype of NK cells producers of IL-4 and IL-5 (NK2 cells)84 which are increased in asthma and atopic dermatitis85, and that in the case of EO could contribute to the histopathological changes observed in the mucosa of these patients65, analogous to the way it occurs in celiac disease86.
B-lymphocytesVery few studies have been aimed at identifying B-lymphocytes in the oesophageal mucosa, although it seems that they could also appear in far lower numbers than T-lymphocytes37. However, cells with intense anti-IgE staining have been identified. These could be plasma cells that secrete this type of immunoglobulin inside the oesophageal epithelium46, that do not express CD19 nor CD20, which are classic B-lymphocyte markers and from which they proceed. Synthesis and local secretion of IgE could justify the existence of symptoms in patients with negative cutaneous tests.
DENDRITIC LANGERHANS CELLSDendritic cells are professional antigen-presenting cells that have the capacity to stimulate naive T lymphocytes and induce primary immune responses or tolerance87. Langerhans cells derive from the bone marrow88,89 and are located in all squamous epithelium90, including the oesophagus, where they can be identified through electronic microscopy91 and immunoperoxidase92, with half the density observed in the epidermis93. Characterised by their CD1a expression94,95, Langerhans cells possess a different identity than monocytes-macrophages, being ontogenetically closer to lymphoid dendritic cells, with functions in antigen presentation, which they catch in the skin and mucosas, internalising, digesting, and transporting them to the lymphatic nodes96, where they present a small fragment of the antigen on its surface to the T lymphocytes in conjunction with the HLA-DR97 and Ia98 type MHC, participating in antigen-specific T cell responses99. In the oesophagus, Langerhans cells possess the same structure as in the epidermis settling on the suprabasal area and along the papillae of the lamina propria, maintaining a stable position, perhaps because of the action of adhesive molecules. Studies by stereology have established that the density of Langerhans cells in the human oesophagus remains stable even in pathological conditions of this organ46.
MAST CELLS IN THE PHYSIOPATHOLOGY OF EOMast cells are widely recognized as effector cells that trigger the innate response against pathogens following their activation through receptors with high affinity for IgE (FCϵRI), Toll-like receptors100 or via non-immunological mechanisms101,102. They are essential in the defense of the host against helminths, and the main effectors of allergic disorders associated to IgE100 through the release of different mediators stored in their cytoplasmic granules, of which histamine stands out. Histamine takes part in the early allergic responses through the binding to H1 receptors, and through other receptors (H2 to H4) it can modulate the immune response103,104 through its action over dendritic cells and T lymphocytes. Besides their effector function, the function of mast cells as a modulator of T cell mediated responses is being increasingly recognized105, with a central role in orchestrating inflammation106.
In normal conditions, mast cells are present in the oesophageal epithelium, increasing their density in GERD, and especially in EO34,39,45,46,107,108. Recent research which analysed the presence of mast cells as a histological marker also showed that there was evidence of cellular activation only in the case of EO mast cells, in the form of positive immunostaining against IgE and structural modifications demonstrable through electronic microscopy45. Mast cell degranulation-inhibiting drugs have been successfully tested in the treatment of eosinophilic gastroenteritis109,110, and appear to be effective for the control of EO symptoms, although further experience is needed. Increasing evidence highlights the potential function of mast cells in the physiopathology of EO: Mann et al. hypothesized that upon antigen exposure, oesophageal mast cells increase histamine levels and subsequently induce an accumulation of eosinophils in sensitised individuals. Secondarily, eosinophilic chemotactic factors result in further eosinophilic accumulation and degranulation111. On the other hand, some of the proteins contained in the granules of eosinophils, particularly Major Basic Protein (MBP) can induce degranulation of mast cells and production of TNF-α, and as a result, the existence of an interaction between these two cellular types can be proposed, in the form of a feedback loop that increases the inflammatory response112. Other Th2 type cytokines released by mast cells determine an environment that favours allergic inflammation processes100, recruitment of T cells and bone marrow proliferation of eosinophils.
The participation of mast cells in the origin of the motor disorders linked to EO has also been proposed: in a study by means of confocal microscopy on alimentary allergy, mast cells have been identified in the vicinity of the afferent nervous fiber113; their content in histamine, Leukotriene C4, and platelet-activating factor can alter the stability of the membrane potential or induce the direct contraction of oesophageal smooth muscle114. Acetylcholine release induced by histamine may result in smoothmuscle contraction of the circular layers in the oesophageal wall. Indeed, mast-cell activation and increased mucosal histamine levels have been observed in experimental models of oesophagitis115,116.
The activation mechanism of mast cells in EO is not clear; in it, allergic sensitisation is common, since the majority of reported patients present high levels of specific seric IgE against multiple alimentary or environmental allergens28,38. However, only a minority of these cases has a history of anaphylaxis against food117, which suggests that IgE would participate in the physiopathology of EO through mechanisms that are different to the classical IgE mediated mast cells and basophile activation in Type I hypersensitivity reactions. Nevertheless, there is evidence of the development of EO shot through an immediate hypersensitivity reaction after the ingestion of foods in previously sensitised patients with elevated serum levels of specific IgE against these foods118.
EOSINOPHIL FUNCTIONS AND PHYSIOPATHOLOGY OF EOThe recruitment of eosinophils towards the oesophagus is the result of the action of different interleukins and eosinophilotropic chemokines, resulting in the onset of the histopathological characteristics of the disease. Eosinophil leukocytes are functionally complex cells, which intervene in the pathogenesis of multiple processes, especially in the protection against parasites119,120 and in allergic reactions56. Their effector function is exerted by means of the preformed cytotoxic proteins stored in their granules (Major Basic Protein, Eosinophil peroxidase, Eosinophil derived neurotoxin, Eosinophil Cationic protein) and lipid mediators (platelet-activating factor, leukotriene C4) that induce the activation of vascular endothelium and contribute to cellular dysfunction22. Eosinophils can act like antigen-presenting cells after being induced to express molecules of the Class II MHC121 and co-stimulating molecules like CD28, CD40, CD80 (B7.1), and CD86 (B7.2)122,123, stimulating, by themselves, T lymphocytes and triggering antigen specific immune responses in vivo. Finally, they have the capacity to secrete lymphocyte-stimulating cytokines, such as IL-2, IL-4, IL-6, IL-10 and IL-1224,124. Furthermore, they exert a proinflammatory effect through the release of a series of cytokines (IL-2, IL-4, IL-5, IL-12, IL-16), chemokines (RANTES and eotaxins), and lipid mediators22, and profibrogenic effects mediated by TGF α/β.
The cytotoxic role of eosinophils in EO is directly related with the observed histopathological changes in the mucosa of the organ, which are characterised by acanthosis, papillomatosis, hyperplasia of basal cells, and spongiosis125,126, with destruction of the most superficial epithelial layers (in contact with the lumen of the oesophagus) and the regenerative response from the basal layers of the epithelium. Correlation between the severity of the histological and endoscopic damage and density and activation of the eosinophilic infiltrate has been described127, although the participation of other cellular types in its generation should be contemplated. At the same time, eosinophils themselves can contribute to oesophageal motor disorders which clinically characterise EO, through the action of MBP as a powerful agonist of the M2 receptors of acetylcholine that govern the function of the smooth oesophageal muscle35,128. In asthma, eosinophils are implicated in the remodelling of the bronchial wall through the release of toxic mediators from its cytoplasmic granules57. Similarly, fibrous oesophageal remodelling in children with EO, by subepithelial collagen deposits, through a mechanism dependent of TGFβ has recently been described129,130.
The action of eosinophil leukocytes over the components of the oesophageal epithelium or over the inflammatory cells themselves can contribute to the maintenance, recruitment, or even the perpetuation of the inflammatory infiltrate. Nevertheless, all the results exposed until now are limited by the fact that they are obtained through the analysis of cellular populations and the cytokine expression at the oesophageal epithelial level, and predict nothing of the behaviour of the inflammatory infiltrate in the subjacent layers, like the submucosal, which is precisely the zone of the organ where the density of the cells with immunological capacities, is greater in normal conditions.
ANTIGENIC SENSITISATION IN EONowadays there is no doubt about the allergic and chronic nature of EO, with an inflammation pattern and a profile of cytokine secretion similar to that found in allergic diseases of the respiratory ways131 and the skin132, which respond satisfactorily to treatments effective in asthma50. EO seems to comprise a different local allergic expression inside the general atopic constitution in these patients2,133, which has special physiopathological implications through mechanisms that have not been totally explained. The number of increased eosinophils in blood in many patients in response to a stimulation of the bone marrow134, the evidence of the inflammation in other organs (like bronchial asthma, rhinoconjunctivitis, or atopic dermatitis), or the antigenic sensitisation throughout different ways, support this hypothesis. Despite the fact that EO can be considered a form of allergy of the digestive tract13,17,135, today we know that sensitisation is not only produced through the oesophageal mucosa: Besides the Mishra et al.42 murine experimental model that developed EO after the antigenic exposure in the upper respiratory ways, the sensitisation against environmental inhalants is common in a large proportion of EO cases. The parallel evolution of the eosinophilic infiltrate with environmental exposure to pollen in sensitised patients has also been reported136,137, with the possibility of exacerbation of its symptoms during the pollination period136,138. On the other hand, sensitisation through the skin has also been demonstrated and the subsequent developing of EO in sensitised mice through an epicutaneous via, by an IL-5 dependent mechanism63. These sensitisation mechanisms could be framed in the concept of "atopic march"139, according to which, the re-exposure to an antigen through the oesophageal mucosa would lead to the activation of the sensitised T-lymphocyte clones and to local inflammatory phenomena, which could be efficiently controlled by treatment with local action topical steroids.
In aeroallergen-sensitised patients, food sensitisation is also frequent140, a fact presented in the literature in more than 50 % of patients with EO. It seems logical to think that a clear relation exists between both types of sensitisation, since foods of vegetable origin can easily present crossed reactions with pollen; this has been observed especially in cereals like wheat, rye, and with grass pollen28. Among food allergens the vegetable based ones; milk; and eggs stand out as the most common. Regarding allergenic sensitisation, while food sensitisation is more frequent in children33,50,141, it seems to be that inhalants could play a more important role in adults: A recent study showed a frequent sensitisation of peripheral blood lymphocytes in adult patients with EO against multiple environmental allergens, in which house dust stood out38, without needing elevated seric IgE to be present against such allergens. In this way an immunological pattern for EO was established depending on the age of patients. More studies that ensure these potential differences are required.
The increase in the incidence of alimentary allergy manifestation and the rising number of possible triggering antigens13 highlights the importance of studying allergic sensitisation in these patients142,143. Diagnostic tests have traditionally been based in the detection of specific IgE against certain foods and inhalants, based on their detection and quantification in serum, or with immediate hypersensitivity skin tests or prick tests. Both of these types of studies show multiple sensitisations in patients with EO141,144,145. Elemental diets (lacking antigenic capacity) have been shown to be an effective solution to EO in children, but the exclusion of foods with an existing IgE-mediated sensitisation does not offer the same result, which leads us to consider the need for cell mediated retarded sensitisation detection. In spite of the frequent presence of elevated serum IgE levels which are specific against multiple allergens in most EO patients, these usually lack a history of anaphylaxis in relation to food, although they can rapidly develop oesophageal symptoms and eosinophilic infiltration following exposure to antigens118,145, especially by oral way. The presence of the complete allergen is necessary for the induction of immediate IgE-mediated allergic responses, while the activation of T-lymphocytes only needs the presence of concrete peptides146. Peptides that do not contain epitopes of recognition for IgE, but which preserve the ones recognized by T-cell, are generated during the digestive process147. It has been proposed that these digestive peptides are capable of inducing strong inflammatory responses mediated exclusively by T-cells148,149, both local and systematic, in the absence of previously IgE mediated events, when faced with re-exposure to the antigen in the oesophageal mucosa, sensitised lymphocytes would organize the inflammatory response by eosinophils, without the participation of IgE80.
The study of atopic diseases conditioned by a cellular immunity disorder has offered greater complications than that of humoral type hypersensitivity manifestations. The methods to analyse cell-mediated retarded hypersensitivity reactions (Patch tests) have been applied to atopic dermatitis (an IgE and non-IgE mediated mixed ethology disorder)150,151 determined by different foods (especially milk, wheat, and nuts152) and, very recently, to EO145, with not very conclusive results; in this last study, the elimination of foods to patients who showed sensitisation, demonstrated through prick test and/or patch test carried out on patients with EO, was so extended that it supposed a real risk of nutritional deprivation153, although it obtained the control of the oesophageal inflammation in a good proportion of cases. The analysis of Th2 profile cytokine's production capacity by peripheral blood lymphocytes in patients with EO when they were incubated in vitro with certain allergens38 represents a new strategy for the study of cell-mediated responses in the study of food allergy.
Besides the control to antigenic exposure of foods or inhalants, considering the previously mentioned data, for a good control of EO it will be necessary to optically control all the allergic manifestations that the patient presents, such as asthma or atopic dermatitis, in addition to the food allergies.
CONCLUSIONSThe complexity and heterogeneity of the data presented possibly expresses the interindividual heterogeneity in the implicated molecular mechanisms in the physiopathology of EO, wherein IL-5 and different types of eotaxins would present synergic effects between themselves and with other less studied cytokines in the regulating of gastrointestinal eosinophil levels41. Even though the final inflammatory phenomena observed in EO are common to different patients, the cascade of inflammatory mediators that lead to them might not be identical in all cases, and the morphological and functional disorders observed in EO would represent the final convergence of different activation forms of inflammation. Likewise, we should consider that the heterogeneity of the results could be related with the time of evolution of the disease or with the moment of the last exposure to the allergen responsible for the epithelial inflammation. These questions should be elucidated through subsequent studies.