There is increasing evidence that oxidative stress is involved in the development and severity of bronchiolitis obliterans occurring in post-transplant patients. In developing countries, the most common form of bronchiolitis obliterans occurs after severe lung infection, mainly caused by adenovirus. However, the oxidative status in the lungs of children with post infectious bronchiolitis obliterans is unknown.
MethodsThe aim of this study was to measure the oxidant (8-isoprostane and protein carbonyls) and antioxidant (catalase and glutathione peroxidase) activity in the bronchoalveolar lavage fluid of 21 children with post-infectious bronchiolitis obliterans, and to correlate oxidant/antioxidant level with lung function. Lung function was assessed by spirometry and plethysmography, one week prior to fiberbronchoscopy.
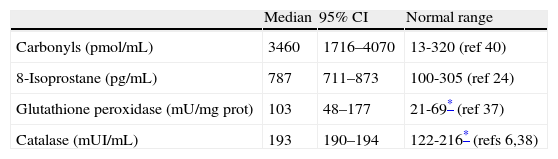
ResultsThere was a markedly increased oxidative stress (lipid and protein oxidation) in the bronchoalveolar lavage fluid, and a notorious impairment of lung function demonstrating moderate-severe distal airway narrowing. There was not a significant correlation between the level of oxidants or antioxidants and lung function. There was a consistent antioxidants/oxidants pattern characterised by markedly increased 8-isoprostane and carbonyls, increased GPx and normal catalase activity.
ConclusionThe present study shows for the first time that children with post-infectious bronchiolitis obliterans have a markedly increased oxidative stress in their lungs.
Lung tissues, and particularly airway epithelium, are in direct contact with oxidants in ambient air and are susceptible to the action of reactive oxygen species (ROS) which could result in damage and inflammation in lung and airways.1–3 On the other hand, normal lungs have effective antioxidant mechanisms to prevent or compensate the effect of ROS.1–4 Although the complexity of mechanisms that control the oxidant/antioxidant balance in the human lung is far from being understood, there is evidence suggesting that oxidative stress (OS) in lung and airways is the result of an imbalance between oxidants production and functioning status of antioxidant systems that may be genetically modulated resulting in differentiated responses. Some polymorphisms of antioxidant genes (glutathione S-transferase) have been found to be associated with enhanced susceptibility to accelerated decline of lung function in smokers;5 whereas in asthmatic airways, oxidative reactions result in catalase inactivation which could amplify OS and so contribute to the chronic inflammatory state.6 It is known that OS stress is invariably present and can contribute to the pathogenesis of several lung diseases evolving with airway inflammation, including asthma; COPD; cystic fibrosis; chronic neonatal lung disease; and also in diffuse interstitial lung diseases, idiopathic pulmonary hypertension, among others.1-4,7
In patients who developed Bronchiolitis Obliterans Syndrome (BOS) as a manifestation of graft rejection,8 the high level of OS found in those patients OS could play a role in the development and progression of the disease.9,10 In developing countries, post infectious bronchiolitis obliterans (PIBO) usually occurs after severe viral pneumonia, mainly cause by adenovirus, and is one of the most frequent causes of severe chronic obstructive lung disease in children in South American areas at the south of the Tropic of Capricorn. The disease usually progresses with important lung structural damage, marked lower airways narrowing, inflammation and impairment of lung function but apparently, its evolution would be more benign11 than post-transplant bronchiolitis obliterans (BO) which remains a major impediment to long-term outcome after lung transplantation.8 At present, it seems there is no information on the OS status in the lung of children with PIBO, or whether the level of OS markers correlates to lung function in those patients. The aim of this study was to determine the level of OS in the bronchoalveolar lavage fluid (BALF) of children with PIBO, and to explore its relationship with lung function.
MethodsTwenty-one children (15 males), with a mean age of 12.6 years (range 8 to 18 years old) with PIBO, without chronic supplemental oxygen in the last five years and who were periodically followed up at the Hospital-CRS El Pino's paediatric respiratory outpatient clinic, in Santiago, Chile, participated in this study. The criteria used for accepting the diagnosis of PIBO were based on previous reports,11 and included history of acute and severe pneumonia associated to adenovirus, permanent airway obstruction after the acute event, imaging findings of chronic lung disease (HRCT with mosaic pattern and air trapping) and exclusion of other chronic lung diseases that have persistent respiratory symptoms.
Lung function testingLung function was measured by spirometry (FVC, FEV1, FEF25-75%, FEV1/FVC) and plethysmography (ITGV, RV, TLC, RV/TLC, sRaw), following international recommendations for acceptability and reproducibility and when patients had been free of respiratory exacerbations for at least three weeks. Bronchodilators were withheld 12 hrs and inhaled corticosteroids were maintained as prescribed. All children were well familiarised with plethysmographic and spirometric manoeuvres, both tests were performed using Master Screen Body® equipment with software version 4.3 (Jaeger®, Germany); the equipment was calibrated daily using a three-litre syringe, and measurements were electronically corrected to BTPS. Reference values and equations employed were Knudson's for spirometry and Zapletal's for plethysmography.12,13
Bronchoalveolar lavage (BAL)BAL was performed under general anaesthesia. Following topical application of lidocaine to the vocal cords, a flexible bronchoscope (Pentax FB10X) with a distal outside diameter of 3.5mm was introduced into the lower airway through a laryngeal mask and wedged in a segmental bronchus of the right middle lobe or lingual. BAL was performed by instilling three aliquots of 1ml/kg of sterile non-bacteriostatic normal saline each, at room temperature and through the suction channel of the bronchoscope. The injected fluid was immediately aspirated using the same syringe over 10–20seconds and only the third aliquot was employed for OS biomarkers assays. The BAL fluid (BALF) samples were centrifuged at 200g during 5min at room temperature and a sample of 500μL of the supernatant was frozen at -70°C until assayed.
BALF assays for oxidants and antioxidantsTotal protein concentration, 8-isoprostane, carbonyl protein, glutathione peroxidase, catalase activity, and superoxide dismutase were measured in BALF supernatant. All measurements were made in triplicate. Total protein concentration in 10μL BALF were quantified by modified Bradford method14 using bovine serum albumin (BSA) as standard. Catalase activity was determined using 10μL BALF by measuring the exponential decay of H2O2 in potassium phosphate buffer monitored at 240nm.15 The 8-isoprostane concentration was measured using a specific enzyme immunoassay kit (Cayman Chemicals; Ann Arbor, MI) in 50μL of BALF.16 The glutathione peroxidase (GPx) activity was measured in 20μl BALF samples with the specific enzyme immunoassay kit (Cayman Chemicals, Ann Arbor MI.).17 Protein carbonyls were estimated using a DNPH-based procedure reported previously.18 The superoxide dismutase (Mn-SOD) activity was assayed in 20μl BALF in potassium phosphate buffer with diethilenetriaminepentaacetic acid.19
Data analysisDescriptive statistics, non-parametric comparisons and Spearman correlation were used for comparisons and associations between lung function values and OS parameters. The results for oxidant/antioxidant measurements are expressed as median and 95% confidence interval (CI) whereas for lung function measurements are expressed as mean and 95% CI; statistical significance was set at a p value <0.05.
The study was approved by the local Ethics and Research Committee and parents signed a full-informed written consent.
ResultsTwenty-one patients completed the study procedures, and no complication due to anaesthesia, fiberbronchoscopy or BAL was reported. All patients had been on treatment with inhaled regular long-action beta 2 agonist plus corticosteroid (≥800 mcg budesonide) at the time of the study.
BALF supernatant assay for ROSMedian total protein concentration in BALF was increased in 18 patients (0.90, 95%CI 0.47-1.03) and normal (≤0.17mg/mL) in three patients; no correlation was found between the level of total proteins in BALF and oxidant/antioxidant concentrations. The median values of oxidant activity (8-isoprostane and protein carbonyl) and antioxidant (catalase and GPx) found in BALF are in Table 1. Both of the oxidant markers (8-isoprostane and protein carbonyls) were highly increased in all patients, whereas antioxidant enzymes activity was normal for catalase and increased for GPx. As expected, no activity of Mn-SOD (0.00 pmol/mL BALF) was detected in the studied samples because of the acellular nature of BALF supernatants. No significant difference was found between males and females regarding OS values or lung function. Moreover, no association was found between oxidants and antioxidant activities.
Oxidants and antioxidants measured in BALF of children with PIBO.
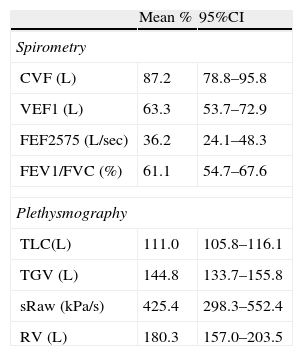
There was not a significant correlation between the level of total proteins or oxidants/antioxidant in BALF and lung function. Lung function parameters were consistently impaired in all patients excepting for FVC and TLC which were within normal limits; values for the studied lung function parameters are in Table 2.
Lung Function measurements as percentage predicted (mean and 95%CI) in 21 children with PIBO.
| Mean % | 95%CI | |
| Spirometry | ||
| CVF (L) | 87.2 | 78.8–95.8 |
| VEF1 (L) | 63.3 | 53.7–72.9 |
| FEF2575 (L/sec) | 36.2 | 24.1–48.3 |
| FEV1/FVC (%) | 61.1 | 54.7–67.6 |
| Plethysmography | ||
| TLC(L) | 111.0 | 105.8–116.1 |
| TGV (L) | 144.8 | 133.7–155.8 |
| sRaw (kPa/s) | 425.4 | 298.3–552.4 |
| RV (L) | 180.3 | 157.0–203.5 |
Spirometry: FVC=forced vital capacity; FEV1=forced expiratory volume in the first second; FEF25-75%=forced expiratory flow between 25% and 75% of FVC. Plethysmography: TLC=total lung capacity; TGV=thoracic gas volume; sRaw=specific airway resistance; RV=residual volume.
No correlations were found between number of years suffering from the disease and spirometric/plethysmographic parameters or oxidant/antioxidant levels in BALF.
DiscussionThis study shows increased OS in the airways of children with PIBO. The markedly increased levels of 8-isoprostane and protein carbonyls in BALF indicate there is augmented lipid and protein oxidation in the airways of these patients. In addition, the antioxidant activity was characterised by normal catalase and increased GPx. This characteristic of antioxidant activity in the BALF in our patients is rather surprising as a sort of common action of catalase-GPx would have been expected. However, a differentiated antioxidant response has also been found in other lung diseases with increased OS, such as idiopathic pulmonary arterial hypertension (normal catalase and decreased GPx)20 and lung cancer (decreased catalase and normal GPx).21 Increased OS is present in several chronic lung diseases such as COPD, asthma, cystic fibrosis, interstitial lung disease, malignancies, neonatal chronic lung disease; it also has been found to occur in passive tobacco exposure and outdoor air pollution suggesting that OS plays a significant role in inflammation, airway narrowing, lung damage and severity of diseases, at all ages.5,6,20–27
Patients with PIBO usually progress with permanent and often severe small airways obstruction, lower airway inflammation, and marked decrease of lung function.11,28 In the present study the level of oxidant or antioxidant enzymes in BALF was not related with any of the lung function parameters studied, suggesting that OS could be more related to airway inflammation and airway hyperresponsiveness (AHR) than to airway calibre. The latter would be supported by the finding that AHR to methacholine which is consistently present in children with PIBO is usually not accompanied by AHR to adenosine 5’-monophosphate,29 which would imply a different underlying mechanism for AHR in patients with PIBO. The lack of correlation between OS (assessed by EBC) and baseline lung function has also been found in asthmatic children,30 but in the same study baseline 8-isoprostane concentration was correlated with a reduction in FEV1 five minutes after exercise, which would point to a closer relationship of OS with AHR than baseline lung function.
In adults, and at general population level, the evidence for the association of OS markers in plasma with pulmonary function is limited and conflicting.31,32 We did not find direct information on this respect (relationship between OS and lung function) in children. The same discrepancies have been reported in patients with chronic obstructive lung disease. While some authors have found antioxidant/oxidant status in plasma to be associated with chronic airflow limitation25 and the severity of obstructive lung impairment,22 others have found no relationship between decreased antioxidant capacity in plasma and airflow limitation in either smokers or patients with COPD.23
There is extensive information supporting that OS is related with airway inflammation in asthma;1–3,6,24,33 however, the exact pathogenic role of OS in the development of allergic or non-allergic airway inflammation, and AHR, is still largely unknown. Experimentally, increased OS in the mouse lung would precede other phenotypes of allergic airway disease, suggesting a critical role for increased OS in the induction of allergic airway inflammation.34 Furthermore, an increasing body of information suggests that active pro-inflammatory mediators as ROS and reactive nitrogen species would be responsible for airway inflammation in asthma.33 Nevertheless, information from standardised longitudinal studies on the role of OS as responsible for airways inflammation in asthmatic and non-asthmatic airway inflammation as well as in AHR modulation and lung function progression, is still lacking.
There is evidence suggesting a genetic modulation of the oxidant/antioxidant balance which could result in an increased production of ROS or in a failure in the upregulation of antioxidant genes, both leading to higher OS. It has been reported that asthmatic children with the genotype for glutathione S transferase (ile105val variant of GSTP1 val/val) had higher systemic OS and lower glutathione levels compared with other genotypes.35
We found a consistent combination of high level of oxidants (carbonyl and 8-isoprostane) with normal catalase and increased GPx activity. This pattern was present in most of our children disregarding the time elapsed since the viral lung injury or the degree of airway narrowing, and might suggest that this oxidative/antioxidant feature may have been set soon after they suffered the adenoviral lung injury. However, it is an open question as to whether this oxidant/antioxidant pattern is restricted just to patients with PIBO. As speculation, it might be that patients with low or absent antioxidant enzymes in their BALF may have a different progression than those with normal levels of catalase or increased GPx as found in the present study. Unfortunately, and despite the increasing importance given to OS in the genesis and maintenance of pulmonary inflammation and damage in several lung diseases, there is still no consensus on what the types of antioxidant/oxidant biomarkers to be measured are, or on the methods that should be employed to assess the oxidative/antioxidative status in BALF or EBC, making comparisons between studies unfeasible.
In our patients with PIBO the markedly increased OS may be related with the severity and progression of the disease. It has been reported that excessive OS and a lack of glutathione are associated with BOS after heart-lung transplantation and with the development of this disorder;9,10 in those patients, OS would be a major risk contributing to the development of the disease and to its ominous progression by inducing more tissue injury and inflammation.10
Increased OS has also been found in infants passively exposed to tobacco smoke during pregnancy or at home.26 Furthermore, active or passive maternal smoking during pregnancy has been found to be associated with important alterations in the balance of oxidants and antioxidants in foetal cord blood causing OS.36 In premature infants, OS is associated to the severity of lung disease suggesting a link between OS and the pathogenesis of infant chronic lung disease.37 It is widely accepted now that OS is present in those patients suffering from different lung disease such as neonatal chronic lung disease, asthma, COPD, CF, interstitial lung diseases, malignant lung diseases, and also in those individuals exposed to respiratory injuries such as tobacco smoke and outdoor air pollution.
Our study is limited by the lack of healthy children as controls and thus we employed normal values (range) as reported by other authors.6,25,30,38–40 In general, the information on OS markers in BALF (in either children or adults) is little, varies significantly among authors and with rare exceptions comes from a reduced number of individuals. Regarding the lack of correlation found between OS levels and degree of airway narrowing in our patients, it is quite difficult to obtain solid conclusions because of the nature of this descriptive cross-sectional study, which does not allow for making causal inference. Whether those patients with higher OS in BALF will develop further functional or structural lung impairment in the near future remains to be answered by longitudinal studies on this matter. Nevertheless, the results of the present study may contribute to increase the understanding of mechanisms causing the structural and functional damage in the lung of patients with BO and may be useful when designing therapeutic interventions.
ConclusionsWe have reported for the first time an increased level of OS markers in the BALF of children with PIBO which would coexist with a differential activity of antioxidant enzyme levels (normal catalase and increased glutathione peroxidase). Despite the marked airway narrowing found in all patients, their pulmonary function parameters were unrelated to oxidative stress markers. Increased OS may have an important role in the development and progression of PIBO.
Conflict of interestThe authors have no conflict of interests to declare.
FundingResearch Grant from the Department of Research, Science and Technology (DICYT), Universidad de Santiago de Chile (USACH), Chile.
We are grateful to the children and parents who participated in this study; their collaboration and support is sincerely appreciated.