INTRODUCTION
Environmental factors such as indoor and outdoor air pollution, tobacco smoke and early exposure to inhalant allergens play roles in the development of asthma in genetically predisposed children1,2. Strong correlations have been found between asthma and early exposure to house dust mite3,4, pet5-7, and cockroach allergens3,8. However, the contribution of fungi to respiratory allergic diseases is not fully evaluated9, though they are the most diverse particles in the air we breathe.
In a previous questionnaire based study of 682 school children, dampness in the home and stuffed toys in the child's bedroom were significant risk factors for asthma by multiple regression analyses (O.R = 2.61; 95 % CI 1.13 to 6.81 and O.R = 2.18; 95 % CI 1.27 to 3.74, respectively)10. Based on these data, we examined the hypothesis that indoor humidity and mold growth in house are risk factors for asthma. Limitations for our initial epidemiological evaluations were allergy skin prick tests (SPT) were not performed, true humidity in homes was not assessed, and indoor pets, toys, etc. were not verified. These factors integrated into new hypothesis.
MATERIAL AND METHODS
Patients
All files of asthmatic children, aged 4-17 years, who had visited the Allergy Clinic of Trakya University Medical School Department of Pediatrics (Edirne, Turkey) at least once during the previous year, were retrospectively analyzed. The patients were part of a large study to examine allergen sensitivities in Edirne and surrounding 100 km area of European Turkey outside Istanbul (n = 400). Among 104 children skin prick tested residing in the Edirne city center, subset of atopic subjects (n = 50) who agreed to participate in this mold evaluation study were included into the study. The families were living in the same address for a minimum period of twelve months. We identified several sets of siblings in our patient group, bringing the total number of homes that were tested to n = 47. The University's Biology Department was limited to a maximum capacity of 75 home mold level assessments. Therefore twenty-five control children living in the same neighborhoods as the asthmatics were chosen from general pediatric clinic patients. The controls had no allergic syndromes including asthma and had negative allergy skin tests. Some of the 25 control subjects were siblings of asthmatics. They were excluded, leaving n = 23 homes for control evaluations.
Asthma was diagnosed in children by history, symptoms, physical findings, and the presence of reversible airflow obstruction as demonstrated by an improvement in FEV1 of greater than 12 % after bronchodilator usage11. The asthmatic patients were being treated for mild or moderately severe asthma, and the mean duration of symptoms was 4.18 years.
The Ethics Review Committee at Trakya University Medical Faculty approved this study. Written informed consent was obtained from the parents for all the participants.
Skin prick tests
Skin tests were performed with Dermatophagoides pteronyssinus and D farinae, fungi (Alternaria tenuis, Aspergillus mix, Candida albicans, Mucor racemosus, mold mix I- Alternaria tenuis, Aspergillus mix, Cladosporium, Penicillium mix, and mold mix II- Botrytis, Mucor, Rhizopus and Stemphylium), animal dander and feathers (cat, dog, sheep wool and feathers mix), and common grasses, weeds, and trees, histamine, and saline (Stallergenes, France). The reactions were recorded after 20 minutes and a wheal diameter 3 mm larger than the wheal diameter of the negative control was considered as positive reaction. All the children tested had positive control wheals ≥3 mm. None of the cases was under treatment that might modify skin tests, and none of the patients had received specific immunotherapy. Atopy was defined as a positive SPT to at least one of the allergens in the panel. Fungal sensitization was defined as having a positive SPT to at least one of the fungi in the panel.
Questionnaire
An interviewer-administered questionnaire was used to characterize any environmental and behavioral variables that affect the presence of indoor fungal propagules. The questionnaire was administered to the parents while the family visited the hospital for the children's skin-prick testing. The questionnaire included structural items such as age of the house, years of occupation, number of occupants, and number of rooms in the house. The instrument also surveyed environmental controls including method of heating, presence of air conditioning, humidifiers, fans, air circulation, and sun exposure. Construction materials like insulation, flooring, and wallpaper were also noted. The presence of indoor pets, cockroach infestations, carpeting, and plants was also noted. Household vapor production was assessed by noting the presence of aquariums, weekly plant watering and floor mopping frequencies, daily cooking hours, and time spent on laundry and bathing per week. The presence of items in the child's room that tend to gather dust was also ascertained. Residents were asked to include any observation of moisture problems, damp patches, mold or mildew growth, musty odors, and flooding or water damage within the past year. Finally, the family's socioeconomic status was determined.
Home visits and sample collection
To verify reports of dampness or mold, all homes were visited by researchers, blinded to the children's case-control status. The surveyors looked for evidence of (a) water damage and flooding intrusion, (b) visible mold growth in any room, (c) musty odors, and (d) visible dust.
Airborne fungal spores were collected from bedrooms, living rooms, kitchens and bathrooms by exposing a petri dish with Rose-Bengal streptomycin agar. Samples were taken between 01.30 P.M and 02.30 P.M. Petri plates were put on a table 80-90 cm in height during sampling for ten minutes. The residents were asked to keep the windows and doors closed at least one hour before the measurements were taken. After sampling, the petri plates were incubated at 25 °C-27 °C for 6-7 days and the number of colony-forming units (CFU) was counted.
At each sampling date, relative humidity and temperature were recorded by using a thermo hygrometer device (TFA-Dostmann GmbH, Gemany) in children's bedrooms. Three other measurements were made by the parents after training them. Home visits were made between October 2000 and February 2001 (average air temperature of 8.74 °C, relative humidity 78.26 %, with mean rainfall of 37.86 mm).
Statistics
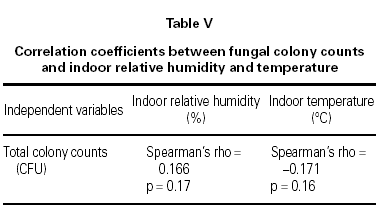
Descriptive statistics are expressed as mean ± SD, median and range. Mann-Whitney U test was used for comparison of the continuous variables. Categorical data were tested using the chi-square statistics. Correlation between fungal colony counts and indoor relative humidity and temperature was performed with Spearman's rho. Residential characteristics related with mold growth were assessed by logistic regression analysis (forward conditional method). All statistical analyses were performed using MINITAB release 13.32 statistical software (MINITAB Inc. US).
RESULTS
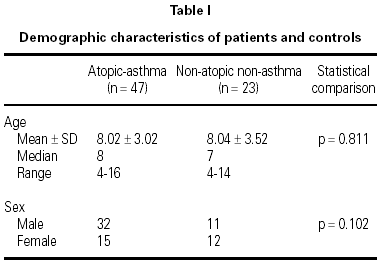
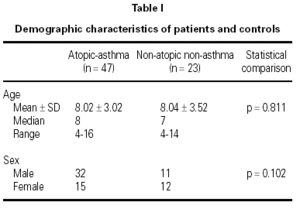
There were 47 children (32 M, 15 F; median age 8) in the asthma group and 23 children (11 M, 12 F; median age 7) in the control group (table I). Of the 47 children with asthma in this study, 35 (74.47 %) had mild asthma, and 12 (25.53 %) had moderately severe asthma. The mean (± SD) duration of symptoms of asthmatic patients was 4.18 ± 3.08 years (median, 4; range, 0.42-14).
Twelve of atopic asthmatic children (25.53 %) were sensitized to at least one of the fungal allergens (or mixture of fungal allergens) that were tested. In control children SPT's were all negative.
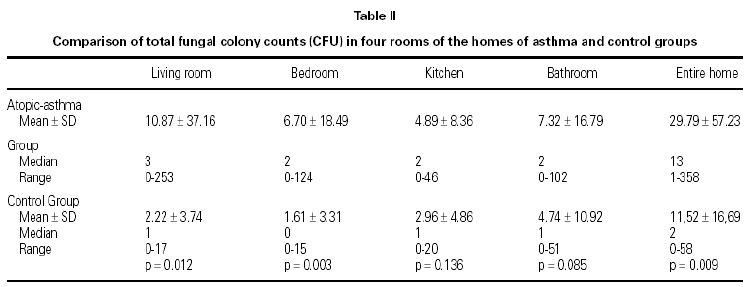
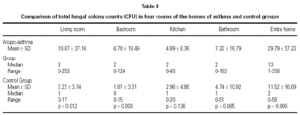
Fungal colony numbers in four rooms (living rooms, bedrooms, kitchens, and bathrooms) from homes of 47 asthmatic and 23 non-atopic non-asthmatic control children are described in table II. The total fungal colony counts in living rooms and bedrooms were significantly higher in asthma group than controls (p = .012, and p = .003, respectively).
All of the petri plates from homes of children with asthma exhibited fungal growth, median CFU = 13. Samples from homes of 16 control cases showed fungal growth; median CFU = 2. The number of homes with total fungal colony counts less than or equal to 2 CFU (median) was 5 in asthma group, and 12 in control group. Total indoor fungal colony counts from entire homes were significantly higher in asthma group than controls (p = .009) (table II).
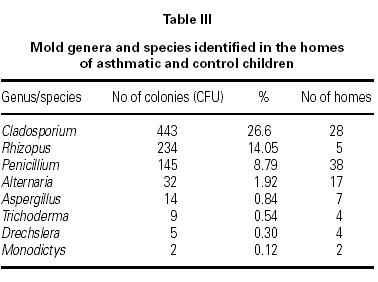
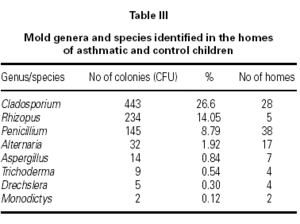
A total of 1665 fungal colonies were isolated in 210 Petri dishes. The rate of unidentified fungal colonies was 31.89 %. The most common isolated genus was Cladosporium, followed by Rhizopus, Penicillium, Alternaria, and Aspergillus (table III).
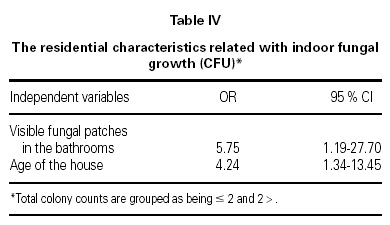
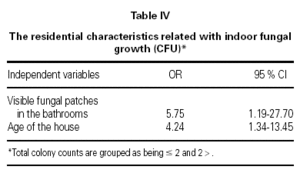
To investigate the associations between indoor fungal growth and environmental and behavioral variables obtained from the questionnaires, and reported or observed signs of dampness, logistic regression (forward conditional method) was used. The total colony counts were grouped as ≤ 2 CFU and > 2 CFU (median as cut off value). Visible fungal patches in the bathrooms (OR = 5.75; 95 % CI 1.19 to 27.70), and the age of the house (OR = 4.24; 95 % CI 1.34 to 13.45) were associated with indoor fungal growth (table IV).
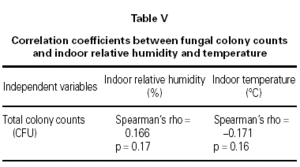
The mean indoor temperature and relative humidity was 22.29 °C and 54.07 % in homes of asthmatic children and 22.39 °C and 50.80 % in controls, respectively. The total CFU counts did not significantly correlate with indoor temperature or relative humidity according to Spearman's correlation calculation (table V).
DISCUSSION
A number of studies from different countries have reported adverse effects of dampness and visible fungal growth in homes on respiratory health. Epidemiological studies that relied on questionnaires12-19 or questionnaires together with visual inspection surveys14,20 found a significant association between home dampness or indoor fungal growth, and wheeze20,16,17, asthma12,13,18 or respiratory symptoms12,14-16,19,20. The severity of asthma13 and mean number of symptoms20 was found to correlate with measures of total dampness and fungal growth.
However, other studies reported that the presence of fungal propagules in indoor air could not be reliably predicted by home characteristics obtained by questionnaires21-23.
In the present study, we investigated the exposure to fungi in indoor environment, in order to assess the role of fungi in asthma. We found total indoor fungal colony numbers to be higher in the homes of asthmatic children than the controls. The difference was especially significant in the children's bedrooms and living rooms, where the children spend most of their times (p = .012 and p = .003, respectively) (table II). Our results suggest that indoor fungal exposure may contribute to childhood asthma.
In few investigations fungus exposure differences between atopic and non atopic children have been addressed. Wickman et al24 observed that concentrations of dust-bound fungi were lower in the homes of atopic children than controls. This observation was explained by intense house cleaning activities of the parents of atopic children. Li et al25 reported that the indoor fungus concentrations of asthma and control groups were higher than those in non-asthmatic atopic children in summer, but there was no difference in indoor total fungus concentrations among the groups in winter26. The presence of Cladosporium correlated with asthma26.
In our study, sampling was performed with open petri dish (OPD), a method which relays on the gravitational deposition of indoor fungal propagules. Even though with this method the quantitative estimation of propagules in a given amount of air is not possible, strong correlations were found between the results obtained with N6-Andersen and the OPD21,27.
The most common isolated genus in our study was Cladosporium, followed by Rhizopus, Penicillium, Alternaria, and Aspergillus (table III). Similar results have been reported in other studies25,27,28. To minimize indoor contamination by outdoor molds we collected samples during late fall and winter season, and while the windows were closed. Our finding of Cladosporium as the most prevalent genus inside homes might be related that our homes do not have sufficient insulation which allows outdoor fungi easily enter indoors.
The skin tests to fungal allergens were found to be positive in 25.53 % of our atopic asthmatic patients. In other studies the rate of skin test sensitivity to common airborne fungal allergens among asthmatic patients were reported to be 2 % to 80 %9,29-34. However, many of the extracts currently available for testing are unstandardized materials, and the true prevalence of sensitivity to fungi in asthma is difficult to establish35. In some of the studies no clear-cut relationship was reported between fungal sensitization and indoor fungal exposure36. However, recent studies indicated that mold related effects were stronger among individuals with specific sensitization to A alternata, C herbarum, or both37. Furthermore, fungi may enhance airway inflammation and cause respiratory symptoms with the production of β-glucans, mycotoxins and volatile organic compounds with a mechanism other than type I allergy33,38.
Damp patches, mold or mildew growth, musty odors, flooding or water damage within the past year, etc. from the questionnaires were not significantly related to the measured culturable fungal propagules in indoor air, in the present study. The only questionnaire found to be predictor of indoor fungal growth was the age of the house (table IV). Older houses are more prone to water problems in combination with insufficient heating and ventilation, resulting home dampness, as reported by Zock et al37 in their questionnaire based survey. In other studies presence of dampness21,24,39, musty odor39, moldy patches39, carpeted floors28,40, household pets28, limited ventilation28,39, or infrequent vacuuming28, were found to effect indoor fungal growth. Our results demonstrate that indoor levels of fungi did not correlate well with the questionnaire reports. Therefore visual inspection surveys should be needed. In our study, mold spots in the bathrooms observed by the researchers was the only factor associated significantly with indoor mold growth (table IV). This suggests that bathrooms were the main source of fungal propagules.
In this investigation, the indoor viable fungal propagules did not significantly correlate with indoor temperature or relative humidity (table V). Ambient humidity may be a poor indicator of conditions in specific indoor microenvironments, where penetrating moisture or condensation on to cold surfaces promotes the growth of moulds24. On the other hand, our measurements were made in the children's bedrooms, however our results suggest that bathrooms were the main source of the fungal propagules.
CONCLUSIONS
Fungal colony numbers were found to be higher in the homes of asthmatic children, especially in the children's bedrooms and living rooms, where the children spend most of their times. Our results suggest that indoor fungal exposure may contribute to childhood asthma.
Indoor levels of fungi did not correlate well with the questionnaire reports. Therefore visual inspection surveys should be needed.
Mold spots in the bathrooms observed by the researchers was the only factor associated significantly with indoor mold growth. This suggests that bathrooms were the main source of fungal propagules.
Although, fungal allergens are thought to be less important in the etiology of asthma than house dust mites, we hypothesize that elimination of household fungi will improve asthma status.
The control of indoor molds requires a concerted approach combining fungicides, measures to reduce home dampness and the removal of mold infested items as soon as possible. Moldy items, such as a basement carpet that has suffered water damage should be removed altogether. Any measures that can be taken to reduce humidity should be recommended. Air conditioners and humidifiers have also been shown to be sources of significant mold exposure. It may be concluded that measures such as frequent application of 5 % bleach into the drainage of the basin and bath-tub, and cleaning visible mold on surfaces can reduce the mold concentration of the bathroom.
The study was carried out at Department of Pediatrics, Trakya University Faculty of Medicine. Edirne. Turkey.
This study was supported by the Research Council of Trakya University (TUBAP-395) (Edirne,Turkey).