INTRODUCTION
The current concept of asthma involves diverse degrees of clinical expression, as expression of complex histopathological and patho-physiological1 base, which lead to an inflammatory process in the bronchial tree. In asthma2, inflammation is a consequence of the embedded participation of a reticular intricate system in which cells, cytokines, chemical mediators, adhesion molecules, etc. participate in response to different types of aggression, which the bronchial tree suffers.
Due to this knowledge, the treatment of asthmatic disease has adapted toward the control of the different processes involved.
During breast feeding and early infancy, various "asthmatic subgroups" were identified: those which developed sibilants following bronchiolitis, another group which possess sibilants with a good general state ("happy wheezing"), usually overweight males and the third group where disturbances in lung function are detected3.
Martínez4 and colleagues describe three clearly differentiated subgroups in early infancy: one linked to viral illnesses, another associated with atopy, and the third with previous problems connected to lung function (chronic obstructive pulmonary disease). In a group of very young children, those episodes induced by virus predominate. Therefore, it is reasonable to believe that the immune situation of the child plays a large role in recovery.
In light of current knowledge, within chemical measurements, leukotriens (LT) play an outstanding role. These are fatty acids derived from arachidonic acid5 which in turn proceed from membrane phospholipids by means of hydrolysis produced by phospholipase A2.
5 Lipoxygenase (5LO), activated by a 5LO activating protein (FLAP), acts on the arachidonic acid leading to a series of intermediate unstable compounds which result in the LT primer LTA4, which in turn is also unstable.
In neutrophils, monocytes and alveolar macrophages activated by LTA 4 hydrolase, LTB4 is obtained with known chemotactic activity and activator of neutrophils, and to a lesser degree, chemotactic of eosinophils.
In cells fundamentally implicated in inflammation, mastocytes, eosinophils and basophils, LTA4 activated by LTA4 synthase will cause the first of the cysteinil leucotrienes (cys-lt) the LTC4. This undergoes an extra cellular metabolism, which gives rise to LTD4 and LTE46.
The effects of LT are measured by protein G (together with BLT receptor for LTB4 and the receptors of cys-LT): cys-LT1 throughout the entire bronchial tree and cys-LT2 at the venous pulmonary system.
LT produce broncho constriction, exudance of plasma, infiltration of eosinophils, increase in mucuos production, contraction of bronchial smooth muscle, disturbance of respiratory flagellum, proliferation of smooth muscle, and specific action as stimulus of liberation of preformed IL-4 in eosinophils7, stimulus of the synthesis of eosinopoietics cytokines8 (GM-CSF, IL-3, IL-5), increase in expression factor of epidermal growth9 IgF, stimulus in production of collagenase by fibroblasts in the lung10, etc.
An open prospective study was designed in order to evaluate the efficiency of treatment with Montelukast, 4 mg/daily, in the control and functional recovery from bronchial asthma in children between 2 and 5 years of age, attended by consultant paediatricians with primary care, and its possible connection with initial immunoglobin values (IgG).
METHODS
Children of both sexes with a syndrome diagnoses of asthma were included in this study, who attended their paediatricians at health centers. In this age group, we define as bronchial asthma 3 or more episodes of bronchial obstruction in the last year. Exclusion criteria were established as follows: 1) no wish to participate in the study; 2) poor tolerance of medication and/or adverse effects (possible bias); 3) wish to abandon study once started; 4) appearance of another pathology causing suspension of study.
32 patients were originally included. Parents were informed that they could abandon study at any time. One week before treatment began with Montelukast 4 mg (one pill taken nightly but not with meal), the peak flow meter had to be registered morning and night, noting the best of three measurements each time. Instructions were given at the clinic on how to carry out tests using the VITALOGRAPH model (donated by PROFAS lab) and the form was provided for data.
Three programmed consultations were done: inclusion in the study for which parental permission was needed, parents were informed that they could abandon study at any time, as well as how to carry out test and collect variable data. A second consultation was held after 4 weeks and last at 8 weeks. The clinical state of the patient was checked in both, control of peak flow and correct register of data were carried out.
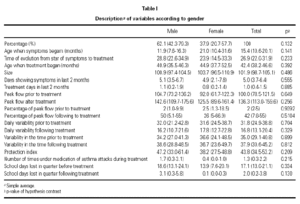
The general variables are: age, sex, height, daily and nightly PEF reading for the 7 days prior to treatment, as well as percentage of immunoglobins (Ig), IgG, IgA, IgM, IgE (presence and type of adverse reaction, evolutionary clinical markers, need for rescue medication, use of CI, days absent from school).
Statistical analysis
We carried out hypotheses contrasts based on non-parametric statistical tests. To contrast the difference between the proportion of men and women in the sample, an exact binomial queue was used. Hypothesis tests on gender difference for quantitative variables were obtained using a Mann-Whitney test for independent samples. For variables related to evolution, (those measures before and after treatment), a Wilcox test of designated ranges was used for matching samples. In the study for association between quantitative variables and non-parametric coefficient of correlation Spearman was used. As for peak flow, the level increase was measured, while in the rest, decrease was quantified in absolute values. For these indicators, the Spearman coefficient of correlation was constructed with the aim of measuring possible relation of improvement with other variables of interest. Finally, we carried out uni-variant regression models for variables of greater evolutionary interest. Statistic package STATA CORP. 200111.
RESULTS
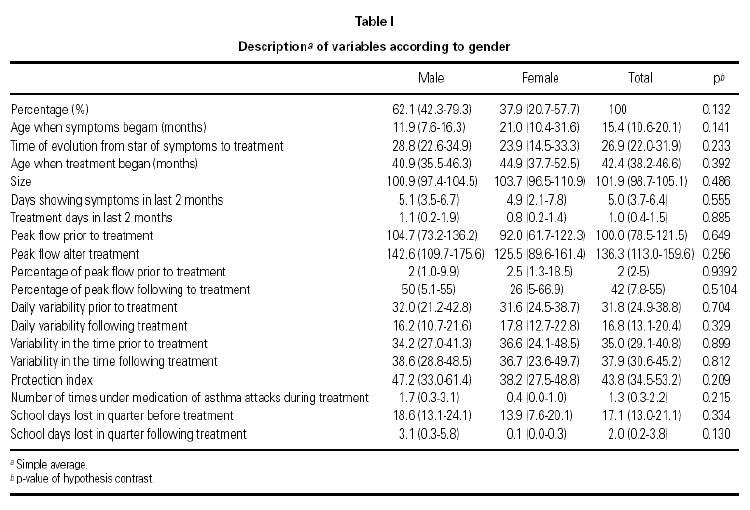
There exists a greater number of male patients (62.1 %) although the difference is not significant. The contrasts in hypothesis concerning distribution of considered variables comparing both sexes did not reveal any significant difference (significance level of 5 %), which suggests that patients have similar characteristics regardless of sex (tables I and II).
Of the 32 patients in the study, 2 did not stard, 1 did not carry out data completion, although the latter did meed some variables. 29 patients began the study. 2 patients abandoned study due to adverse reactions (diarrhoea in one, and nightmares and hives in the other). One other patients abandoned study due to a parental decision, 26 patients completed the study overall. These general results are reason for comment in another article.
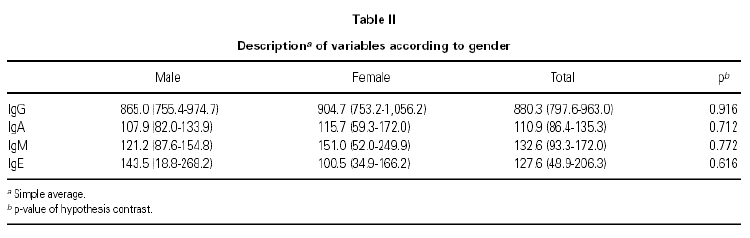
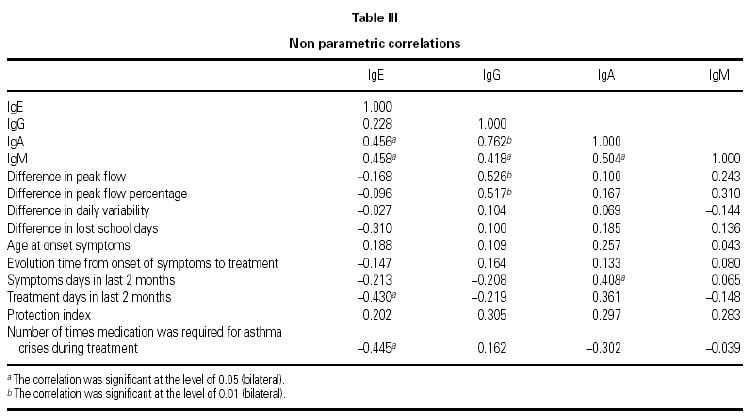
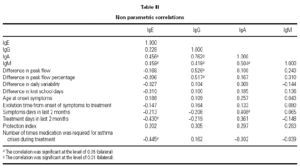
We find a positive link between all Ig pairs, except between IgG and IgE. We also see an important positive link between IgG and IgA values (coefficient correlation 0.762), therefore the greater level of IgG that exists the greater level of IgA will be found and vice versa.
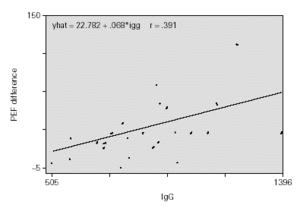
With regard to the PEF value reached after treatment, there exists a positive correlation with IgG values at the beginning of treatment (0.712), and similarly but to a lesser degree, with IgA values (0.660).
Those children with the highest IgG values at the start of the study, obtained the highest PEF value at the end of the 8-week treatment period with Montelukast. The same occurred in relation to the IgA value, but with a lesser correlation.
No correlation between the IgG value at the beginning of the study and the rest of the considered variables was found.
Medication during same (0.445), as if a greater IgE value meant fewer treatment days, compared with children having lower IgE and requiring. The IgE value was curiously associated although negatively with those days when treatment was required 2 months prior to study (0.430), and with the need for rescue less rescue medicine during the study (table III).
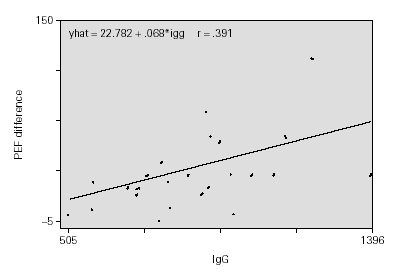
The adjustment of uni-variant linear regression models revealed a possible link between the IgG value and the increase in PEF, finding no significant statistical link with remaining Ig. This link is maintained even when the multi-variant model is adjusted. For each 100 mg/ml of increase in the IgG value, a rise of 10 l/min is produced in the PEF measurement, prior or following treatment, adjusted for time of evolution, protection rate, IgA and IgM (fig. 1).
Figure 1.--Time of evolution from symptom.
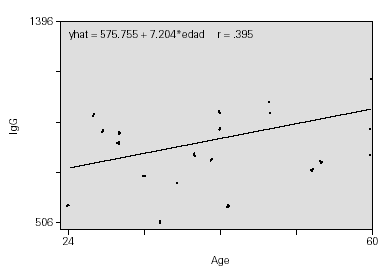
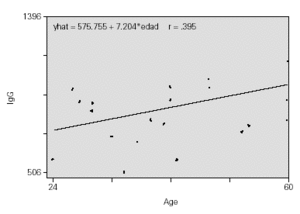
As expected, the IgG values increase with age, with the proportion of 7 mg/ml for each month (fig. 2).
Figure 2.--Protection index.
DISCUSSION
That Ig values increase with age during development is well known12,13. This involves progressive maturation of the immune system, originating in the foetal period and accelerating from then on, in response to contact with a diversity of environmental agents. There is data which supports the existence of certain differences between boys and girls14,15: significantly higher levels of IgM have been found in women14, and even in some cases IgA in a population aged between 10 and 7315.
Children with higher IgG at the beginning of the study had elevated PEF values at the end, i.e. they showed a better functional respiratory response quantified in an increase of 10 l/min in PFM per each 100 mg/ml which increased the IgG value.
A large variety of results referring to IgG values exists as well as sub-classes of IgG, IgA, etc. and their relationship with asthma or recurrent sibilants in early childhood. It is evident that they play some role in the clinical expression of this pathology in the ages considered, however, this role is still as yet unclear.
From a sample of 37 children, Smith discovered lower levels of IgG sub-classes (IgG1, IgG2, IgG4) in intrinsic and extrinsic asthmatic children, whose proportion was higher in children who had lower initial IgG values16, although a normal concentration of IgG in serum does not exclude the possibility of a deficit in sub-classes. Smith suggested the possibility of an etiological role in the respiratory symptoms of some dhildren who showed deficits of the sub-classed IgG mentioned above.
A connection between the IgG sub-class deficiency and bronchitis symptoms in early childhood has recently been found17. This is more frequent in these age groups than later, suggesting a transitory immaturity of immunity. The etiology of recurrences is often viral in these age groups than in later age groups, meaning that in predisposed children a lower IgG "protective" activity could be involved (neutralization, complementary activation and phagocytosis).
In another recent sutdy of 42 children age between 9 months and 6 years, lower levels of IgG4 were found in children under 24 months than in the control group. From 2 to 6 years, IgE was found to be increased, while IgG3 and IgG4 (p < 0.05) were lower compared to the controls18, suggesting a relationship between lower levels of IgG sub-classes and sibilants in early childhood.
In Brazilian children aged 7 to 15, lower IgA levels and sub-classes of IgG were found in the group of severe asthmatics, with an IgG3 deficiency being predominant, although this does not seem to be a reliable predictor for the development of infections in this children19.
In Japanese children the prevalence of deficit in IgG sub-classes was 31.6 % in breast fed children of 6 to 24 months and 26.7 % in controls. There were no significant differences, so the conclusion was reached that sibilants in the breast fed child are not linked to a deficiency in IgG sub-classes, although there exists a link between recurrent sibilants and concentration of IgG sub-classes20.
In older Asian children with asthma (average 7.5 years), no deficiency in IgG sub-classes was found. Only IgE levels were four times higher than control subjects21, suggesting that at these ages there is greater participation of IgE responder as opposed to environmental allergens and a larger maturation of IgG responder.
IgD levels were found to be lower in children with the first signs of atopic asthma (p < 0.001), although these were normal in the course of the following 18 months22. Is this a non- specific response or an attempt to block the immune response in asthma favouring tolerance?
The transitory absence of IgA in saliva during the first years of life is linked to an increased risk of bronchial hyperactivity23 BHR, but no link was found between this IgA transitory deficiency and the clinical diagnosis of asthma, which would favour the thesis of independence of these two clinical frameworks.
IgA and IgD levels were found to be lower both in patients with intrinsic and extrinsic asthma24, compared with the control group. IgA was found quite high in smokers. Women had significantly higher IgA levels compared to men. Both IgG and IgA levels increased with age (14 and 24).
From a sample of 315 apparently healthy males aged between 19 and 22, D'Amelio found no relation between clinical history or IgE levels and the remaining Ig classes25, as occurred in our study, where no link between IgE levels and the remaining IgE classes was found in such low age groups as those of our patients.
Those respiratory and non respiratory pathologies where different deficits of IgG26 sub-classes are found are very diverse and their exact interpretation and comprehension has yet to be fully understood.
In addition, in the broncoalveolar lavage (BAL) in asthmatic patients, the IgG sub-classes were significantly higher than in healthy control subjects27, showing a local production of 1 or more sub-classes and an increased exudance of same.
The predominant Ig in BAL is IgA which seems to be produced locally, finding significant statistics for concentration of sIgA and IgA I patients with allergic rhinitis28. The authors suggest the possibility of some role played by this Ig in the mediation of the atopic illness. When we study the influence of the maternal immune response in a new born baby and in the early years of life we find some data of interest. The Ac. as opposed to the IgE maternal allergens, are consistently linked to the presence of asthma in the mother, while those IgG class Ac. are linked to maternal rhinitis and eczema in the chils29.
Moreover, treatment with intravenous Ig in patients with severe asthma often leads to clinical improvement, reduction in the need for corticoids and a decrease of cutaneous reactivity in prick-test30. Finally, it is important to understand the present situation regarding "hygiene hypothesis" in the development and management of bronchial asthma 31. Good hygiene in the early years is linked to a lesser prevalence of atopic illness. In a study carried out in Denmark on an adult population and considering seropositivity for Hepatitis A, Helicobacter pylori and Toxoplasma gondii as markers of poor hygiene, a link was found between positivity to 2 of these markers and low prevalence of atopy, while colonization of the intestinal tract by potentially pathogenic bacteria is associated with high prevalence of atopy32.
As regards infection by certain pathogens and their relation to atopic illness, new data appears again.
Bjronsson33 et al, find a link between recent infection (IgM > 1/16) and/or previous infection (IgG < 1/512 and > 1/32) per Ch1. Pneumonic and the presence of sibilants. Later, Mill34 et al, did not find any positive link between suggestive symptoms of asthma and the two previous years and Ac. IgG as opposed to C. Pneumonia.
In the population age group of our study where it is known that there exists at least 3 sub-groups of patients with recurring sibilants4, the development of immune response must be an important factor in the clinical evolution of the illness and possibly, at the same time, participate in the genesis of same, if current evidence is taken into account. Those patients whose initial immune state is better would expect to show improved functional respiratory recovery with age considered. Perhaps the neutralization of the virus, which is responsible for reccurrences in early childhood, would be more effective in those children who finally reach greater functional recovery, In these cases, mediation of eicosanoids coming from alveolar macrophages is known, by which treatment with montelukast could have an association with this response.
In any case, it would be useful to design a study with a larger population, greater follow-up period with control in series of IgG concentrations, to evaluate if these could aid prognosis of the disease, either in the short or long term depending on what type of treatment is established.
CONCLUSIONS
The sex of the patient did not influence results. IgG values increase with age being quantified in our case in 7 mg/ml of increase for each month that age increases. The children with the highest IgG values before treatment began reached the highest PEF values after being treated with 4 mg of MONTELUKAST daily for 8 weeks (10 l/min of PEF increase for each 100 mg/ml of increase in IgG value).
The literature does not clarify the mechanisms implicated in these results. There exists various data which certify the implication of these Ig in the asthmatic process, which seems greater in breast fed and small children. Perhaps a possible explanation could be greater protection as opposed to precipitating factors of asthmatic symptoms, of viral and bacterial type, which their association would have greater impact on age groups more affected by these precipitated factors.