The murine polyp model was developed previously using ovalbumin and Staphylococcus aureus enterotoxin B (SEB). Here, we established a model mimicking key aspects of chronic eosinophilic rhinosinusitis with nasal polyps using the house dust mite (HDM), a clinically relevant aeroallergen, co-administered with SEB. We assessed the inflammatory response and formation of nasal polypoid lesions in an experimental murine model using intranasal delivery of HDM and ovalbumin.
MethodsAfter induction of HDM-induced allergic rhinosinusitis in C57BL/6 mice, SEB (10ng) was instilled into the nasal cavity of mice for eight weeks. Phosphate-buffered saline-challenged mice served as control. Histopathological changes were evaluated using haematoxylin and eosin for overall inflammation, Sirius red for eosinophils, and periodic acid–Schiff stain for goblet cells. The distribution of mast cells in mouse nasal tissue was determined by immunohistochemistry. Serum total IgE was measured using enzyme-linked immunosorbent assay.
ResultsCompared to mice treated with HDM only, the HDM+SEB-treated mice demonstrated nasal polypoid lesion formation and a significant increase in the number of secretory cells and eosinophilic infiltration. Moreover, mice challenged intranasally with HDM showed highly abundant mast cells in the nasal mucosa. In contrast, OVA+SEB-challenged mice showed a significantly lower degree of mast cell infiltration.
ConclusionWe established an in vivo model of chronic allergic rhinosinusitis with nasal polypoid lesions using HDM aeroallergen. This study demonstrated that the HDM+SEB-induced murine polyp model could be utilised as a suitable model for nasal polyps, especially with both eosinophil and mast cell infiltration.
Chronic rhinosinusitis (CRS) encompasses a heterogeneous group of disorders defined by the inflammation of the paranasal sinuses and represents a significant health problem worldwide. CRS has been categorised typically as CRS with nasal polyps (CRSwNP) and without nasal polyps (CRSsNP) based on endoscopic examination findings.1,2 CRSwNP is characterised by inflammatory cell infiltration and structural modifications of the epithelium (secretary hyperplasia and squamous metaplasia) and lamina propria (basement membrane thickening, extracellular matrix accumulation and fibrosis). However, the underlying mechanisms interlinking these pathological conditions to nasal polyp formation remain unclear.3
To date, murine studies investigating CRS have employed systemic sensitisation and intranasal challenge with ovalbumin (OVA). Chronic challenge models in mice involve repeated exposure to antigen for up to 12 weeks. Low-dose Staphylococcal enterotoxin B (SEB) induced nasal polypoid lesions with increased eosinophilic infiltration in an allergic rhinosinusitis model.4 OVA challenge models of CRSwNP offer opportunities for increasing our understanding of the pathogenesis underlying this disease, as well as for identifying novel therapeutic targets, although more relevant allergens such as the house dust mite (HDM) may also be considered.5,6
The HDM is ubiquitous in human habitats and is a significant factor underlying allergic rhinitis and allergic asthma. These features make it one of the important sources of indoor allergens.7,8 The predominant HDMs isolated from dust samples are Dermatophagoides pteronyssinus (Der p) and Dermatophagoides farinae (Der f). Although HDM extracts are complex from an immunological perspective, they are ultimately more representative of real-life aeroallergen exposure.9,10 Recently, common parameters of airway allergy such as airway inflammation, Th2 cytokine production and elevated Der f-specific IgE levels were shown in an intranasal HDM sensitisation mouse model.11–13 Despite the high prevalence of HDM allergy, the cellular and molecular networks that initiate and regulate this Th2-biased response have been investigated using only the OVA-induced polyp model.14
In this study, we established a model mimicking key aspects of CRSwNP using the clinically relevant aeroallergen HDM with co-administration of SEB. Additionally, we compared the immune-inflammatory and nasal polyp formation responses to intranasal delivery of HDM extract and OVA in the experimental murine model.
MethodsExperimental animalsEighteen male C57BL/6 mice (4 weeks of age; 20–25g each) were purchased from Central Laboratory Animal, Inc. (Seoul, Korea) and housed for one week before initiating experiments. The animals were kept in a pathogen-free biohazard containment facility maintained at 22–24°C and 50–60% humidity. All experimental protocols complied with the Guidelines of the National Institute of Health and the Declaration of Helsinki, and were approved by the Committee on the Use and Care of Animals (SNU-140429-10).
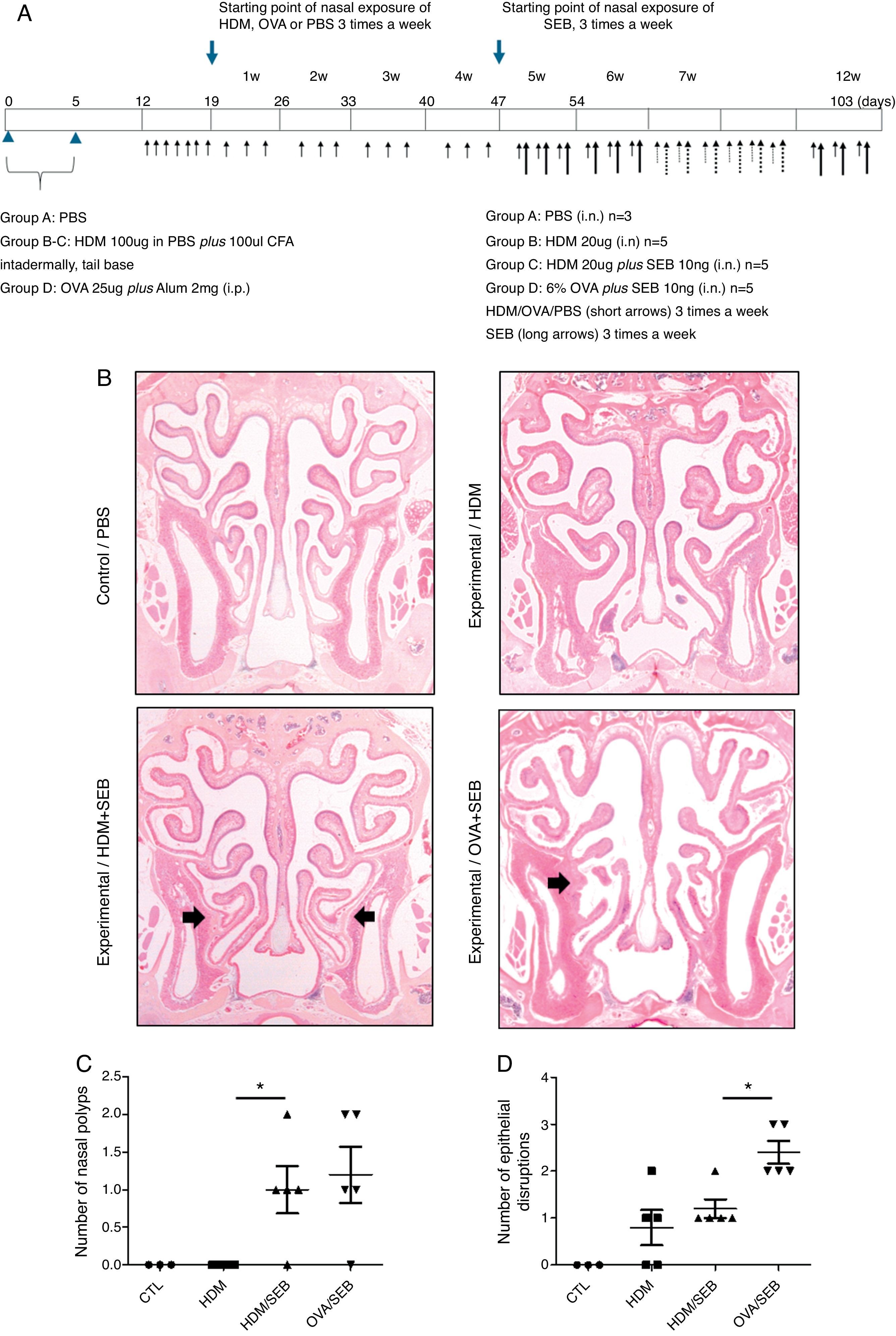
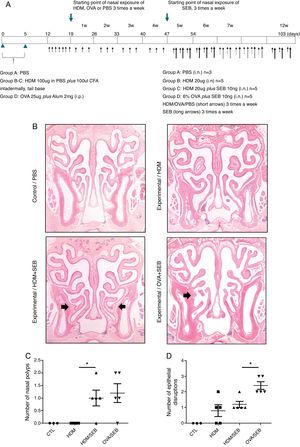
Experimental protocolsThe experimental protocol was designed as described previously with some modifications (Fig. 1A).4,12 The mice were categorised into a control group (group A; n=3) and three experimental groups (B, C, and D; n=5 each). Purified HDM extract from species Der f (GREER Laboratories, Lenoir, NC, USA) was used in this study. Mice in experimental groups B and C were immunised intradermally at the base of the tail with 100μg of HDM extract dissolved in phosphate-buffered saline (PBS) and 100μL of complete Freund's adjuvant (CFA, Sigma, St. Louis, MO, USA) on days 0 and 5. The mice in experimental group D were systemically sensitised by intraperitoneal injection with 25μg of OVA (Grade V; Sigma, St. Louis, MO, USA) dissolved in 300mL of PBS in the presence of 2mg of aluminium hydroxide gel adjuvant. Seven days after the last immunisation, mice were challenged intranasally with 20-μg HDM extract dissolved in 40-μL PBS (groups B and C) or 6% OVA in 40μL of PBS (group D), and treated daily for seven days.
Development of allergic rhinosinusitis with nasal polypoid lesions in C57BL/6 mice. (A) Protocol for allergen sensitisation and administration of Staphylococcus aureus enterotoxin B. HDM, house dust mite; SEB, Staphylococcus aureus enterotoxin B; OVA, ovalbumin; PBS, phosphate buffered saline; i.p., intraperitoneal injection; i.n., intranasal instillation; W, week. (B) Mice in groups A, B, C and D were treated with PBS, HDM extract, HDM extract+SEB (10ng), and OVA (6%)+SEB (10ng), respectively. Nasal polypoid lesions (arrows) were detected only in groups C and D (haematoxylin and eosin stain, H&E). (C and D) Numbers of nasal polyps and epithelial disruptions were compared. *p<0.05, Mann–Whitney U test.
Prolonged continuous inflammation was maintained in the experimental groups by subsequent nasal treatment with 20-μg HDM extract or 6% OVA three times weekly for 12 consecutive weeks. Additionally, selected mice in groups C and D were challenged three times weekly with 10ng of SEB (List Biological laboratories, INC, CA, USA) diluted in PBS from the 5th week to the 12th week. PBS was applied for both systemic and local stimulation in group A (control). Mice were sacrificed 24h after the last intranasal administration.
Histopathological analysisThe mice were euthanised and decapitated. The heads of the animals were stripped of skin, eyes and muscle, and the mandibles were excised. The tissues were immersed overnight in 4% paraformaldehyde and decalcified in 5% nitric acid for 4–5 days at 4°C. The specimens were dehydrated and processed according to standard paraffin-embedding procedures.15 Tissues were then cut into 3-μm-thick coronal sections. An atlas of normal murine sinonasal anatomy was used to standardise the anatomic locations being examined.16 The histological changes in the nasal mucosa were determined by haematoxylin and eosin (H&E) for overall inflammation, Sirius red for eosinophils, periodic acid–Schiff (PAS) stain for goblet cells, and Masson's trichrome stain for collagen deposition in the subepithelial layer.
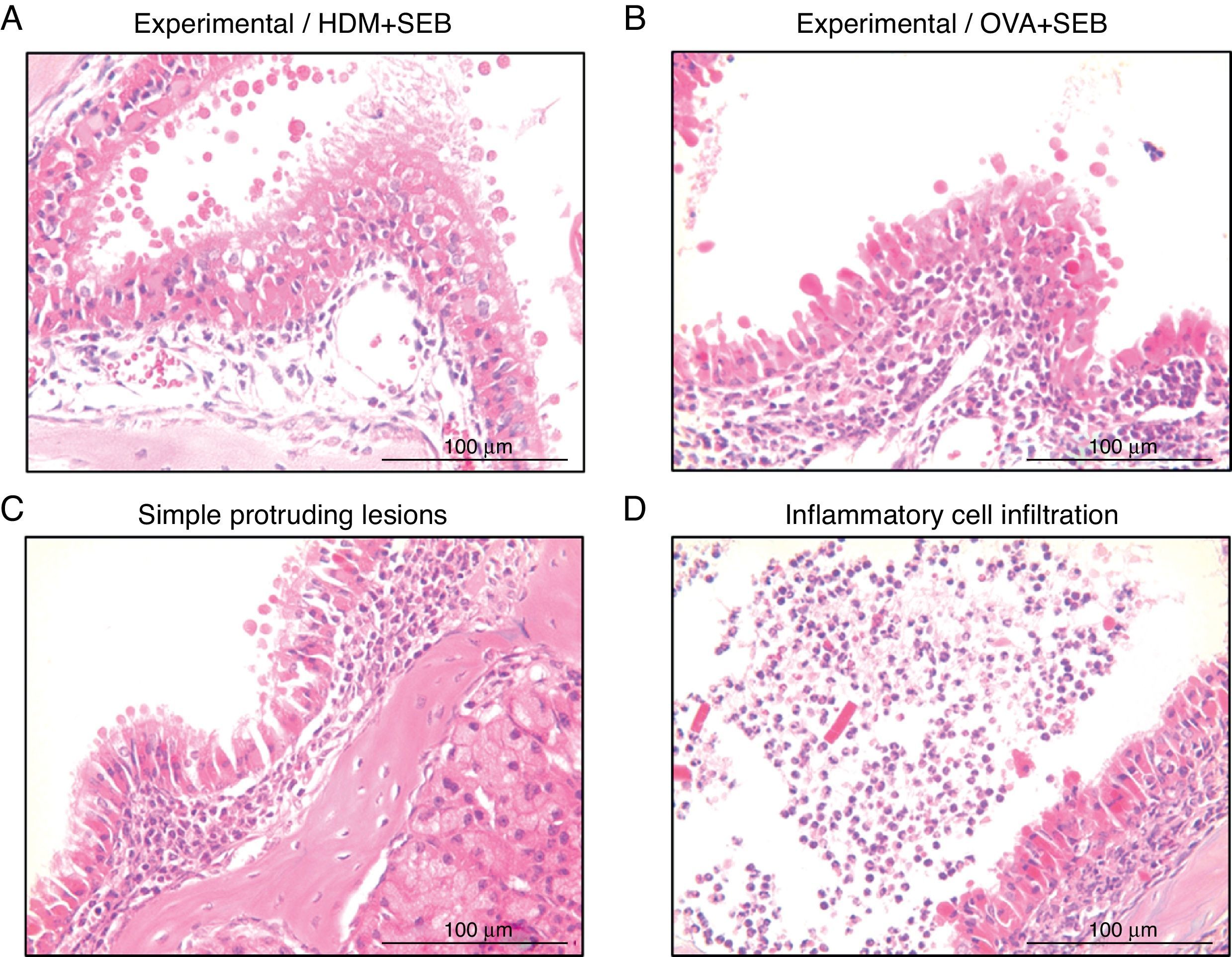
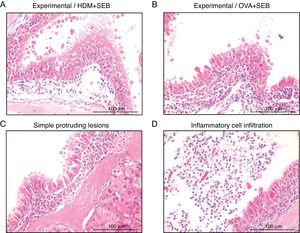
To determine the degree of inflammation, the sections were examined with a light microscope (400× magnification), and two individuals independently counted the number of eosinophils, mast cells and goblet cells in the nasal mucosa in four fields. The results of inflammatory and goblet cells were expressed as cells per high-power field. Subepithelial thickness was measured from four mucosal sampling areas under a high-power field (400× magnification). Three consecutive slides were reviewed to rule-out processing errors. Polyp-like lesions were defined as distinct mucosal elevations with eosinophilic infiltration and/or microcavity formation (Fig. 2A and B). Polypoid lesions and epithelial disruption were counted microscopically and expressed as a total number. Simple protruded lesions were not regarded as polyp-like lesions (Fig. 2C).
Photographs of representative nasal polypoid lesions stained with H&E. Sinonasal tissue sections were examined microscopically to detect polypoid lesions. (A and B) Polyp-like lesions were observed in mice treated with HDM+SEB or OVA+SEB. Polypoid lesions were characterised mainly by the presence of eosinophils and mast cells. Simple protruding lesions (C) and eosinophilic infiltrates with Charcot–Leyden-like crystal formations (D) were also found. Scale bar=100μm.
Immunohistochemistry (IHC) was performed to identify the presence of mast cells. IHC staining was performed using the polink-2+polymerised horseradish peroxidase (HRP) broad DAB Detection System (Golden Bridge International Labs, WA, USA). Briefly, tissue sections were deparaffinised, rehydrated and treated with 3% hydrogen peroxide in methanol to quench endogenous peroxidase activity. Heat-induced epitope retrieval was then performed by microwaving the samples in 10mmol/L citrate buffer (pH 6.0). The sections were incubated for 1h at room temperature with primary antibody against mast cell tryptase (1:100, Abcam, Cambridge, MA, USA). The sections were incubated in broad antibody enhancer and polymer-HRP and then stained with the DAB Detection System. Sections were counterstained with Gill's haematoxylin and dehydrated through a graded ethanol series, cleared with xylene, and coverslipped with mountant. Negative controls were obtained by omitting the primary antibody. To confirm mast cells, Giemsa staining was also performed according to standard procedures.
Quantitative determination of total IgEBlood was collected via cardiac puncture 24h after the last intranasal administration. The blood was centrifuged, and the serum was stored at −80°C for the measurement of IgE. Serum samples from three animals per group were analysed. Quantitative assessments of total IgE were performed using an enzyme-linked immunosorbent assay (ELISA) kit purchased from BioLegend (San Diego, CA, USA). The sensitivity of total IgE was 0.1ng/mL. All procedures were performed according to the manufacturer's instructions.
Statistical analysesData are expressed as means±SD. The Mann–Whitney U-test was performed to compare the number of eosinophils, mast cells, goblet cells and subepithelial thicknesses between the groups. Statistical analyses and data plotting were performed using SigmaPlot (version 10, Richmond, CA, USA). A value of p<0.05 was considered to indicate statistical significance.
ResultsThe formation of nasal polypoid lesionsNo polyp-like lesions were observed in groups A and B (Fig. 1B). Polypoid lesions were found only in mice that received SEB intranasally (groups C and D). Five lesions were observed in three of five mice in group C (Fig. 1C). Similarly, six lesions were evident in four of five mice in group D (Fig. 1D). Thickened mucosae with polyp-like lesions were observed primarily at the transition zone of the olfactory and respiratory epithelia. Morphological changes such as secretory hyperplasia as well as eosinophilic infiltrates with Charcot–Leyden-like crystal formations were observed in the epithelial lining of nasal polyps (Fig. 2). Epithelial disruptions as well as polypoid lesions were found more often in the group treated with OVA+SEB (Fig. 1C and D).
Inflammatory responses in the airwayIntranasal delivery of HDM extract alone or HDM+SEB elicited robust inflammatory responses in the airways of mice, which was clearly evident histopathologically. Therefore, we next characterised the inflammatory cells in the nasal mucosa. The magnitude of the inflammatory response was estimated by the number of inflammatory cells recruited into the tissue.
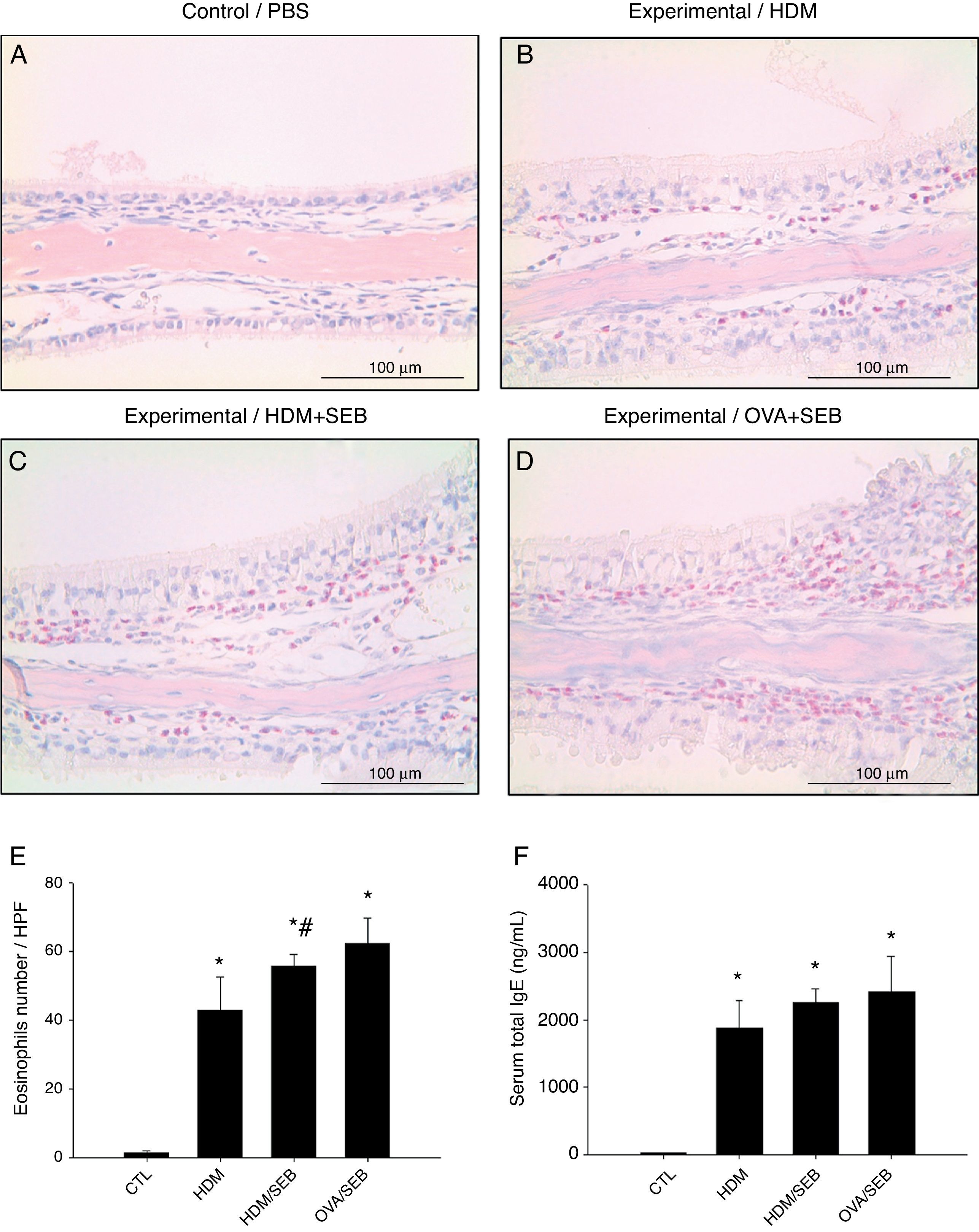
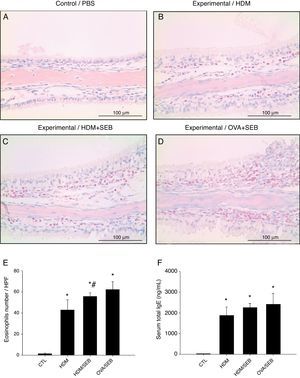
As shown in Fig. 3, HDM only or HDM+SEB treatment induced tissue inflammation in the nasal mucosa, and the inflammatory infiltrate was composed mainly of eosinophils. The numbers of eosinophils per high-power field in groups A, B, C and D were 1.3±0.5, 42.8±9.6, 55.6±3.5, and 62.2±7.5, respectively. Mice challenged with HDM+SEB and OVA+SEB exhibited significantly higher numbers of tissue eosinophils than mice challenged with HDM only (p<0.05), whereas no significant difference was detected between mice treated with HDM+SEB and OVA+SEB. In addition, the total serum IgE levels were elevated markedly in the three experimental groups, with no significant difference among groups B, C and D (Fig. 3F).
Representative sections of eosinophilic infiltration and total IgE production. (A–D) The presence of eosinophils was detected in groups B, C and D. Increased numbers of eosinophils were observed in groups C and D, with no significant difference in eosinophil distributions between groups C and D. (E) The numbers of eosinophils infiltrating the nasal mucosa were counted based on the Sirius red-stained sections. Data are expressed as means±SD. (F) The level of total IgE from serum was measured by enzyme-linked immunosorbent assay (ELISA). Data are expressed as means±SEM. *p<0.05, compared to group A (control); #p<0.05, compared to group B (HDM); Mann–Whitney U test. Scale bar=100μm.
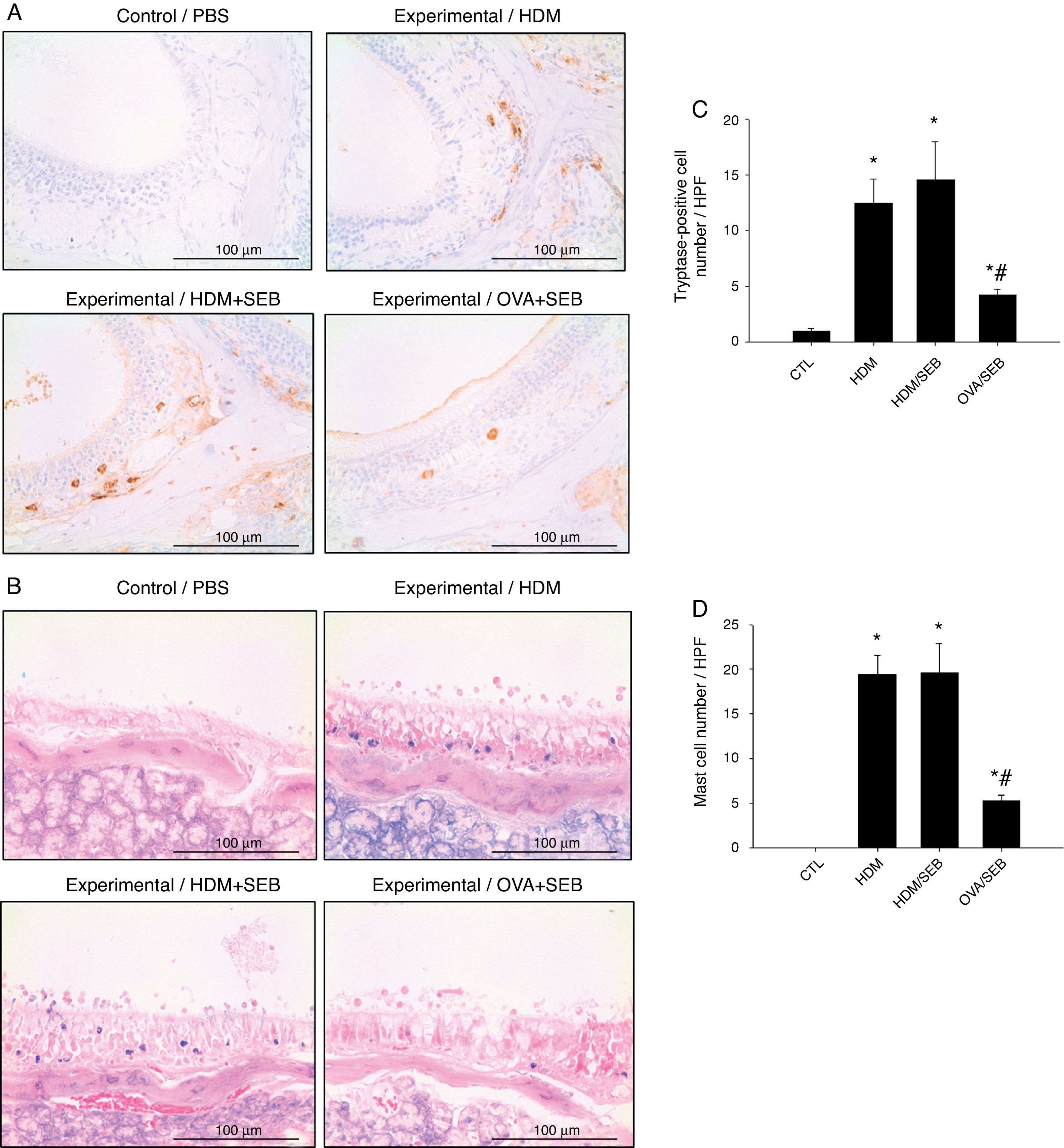
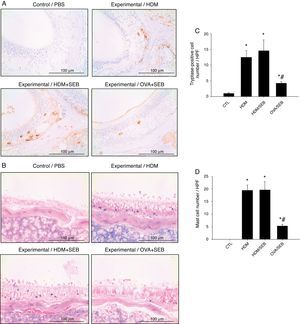
The distribution of mast cells in nasal tissue sample in mice was determined by IHC (Fig. 4). The numbers of tryptase-positive mast cells per high-power field (mean±SD) in groups A, B, C and D were 1.3±0.5, 12.4±2.0, 14.5±3.5 and 4.2±0.5, respectively. Similarly, Giemsa staining of the nasal tissue sections revealed abundant mast cells after instillation with HDM or HDM+SEB, but an absence of mast cells in the airways of PBS-treated mice. The numbers of mast cells per high-power field (mean±SD) in groups A, B, C and D were 0.0±0.0, 19.4±2.1, 19.5±3.3 and 5.3±0.6, respectively. Mast cells were most numerous in the lamina propria but were also frequent in the epithelial layer. We found that after intranasal administration of OVA+SEB, the degree of mast cell infiltration was significantly lower than that in groups B and C (Fig. 4C and D; p<0.05).
Increased infiltration of mast cells in house-dust-mite-induced polyp model. (A) Immunohistochemical detection of tryptase positive-mast cells in the nasal mucosa. (B) Mast cells were identified by their characteristic cytoplasmic granules using Giemsa staining. Mast cells were distributed throughout the lamina propria and also localised within the epithelium. (C and D) Groups B and C showed markedly denser distributions of mast cells than group D. Data are expressed as means±SD. *p<0.05, compared to group A (CTL); #p<0.05, compared to group C (HDM/SEB), Mann–Whitney U test). Scale bar=100μm.
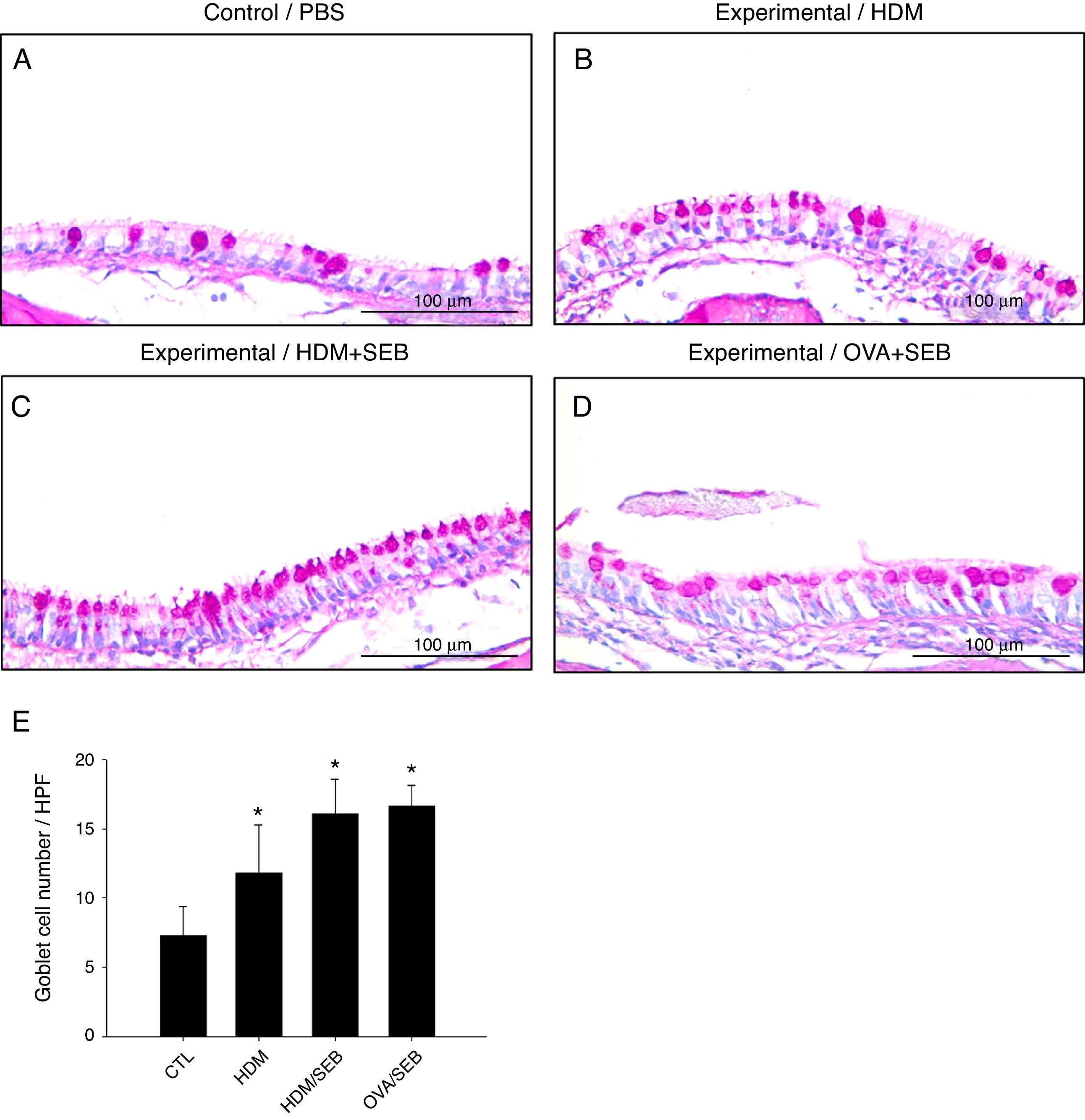
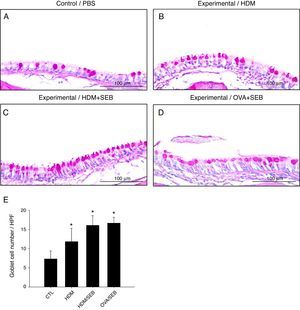
Changes in airway goblet cell numbers in all groups are illustrated in Fig. 5. The numbers of goblet cells per high-power field (mean±SD) in groups A, B, C and D were 7.3±2.0, 11.8±3.4, 16.0±2.5 and 16.6±1.5, respectively. Our results showed that the number of goblet cells of the nasal mucosa increased significantly in the experimental groups (p<0.05). However, no significant difference in the degree of goblet cell hyperplasia was detected between groups C and D (Fig. 5C and D).
Goblet cell hyperplasia in house-dust-mite-induced polyp model. (A–D) Representative photomicrographs of nasal mucosa sections of mice stained with periodic acid–Schiff (PAS). (E) Increased numbers of goblet cells in the experimental groups, especially in groups C and D. *p<0.05, compared to group A (CTL); Mann–Whitney U test. Data are expressed as means±SD. Scale bar=100μm.
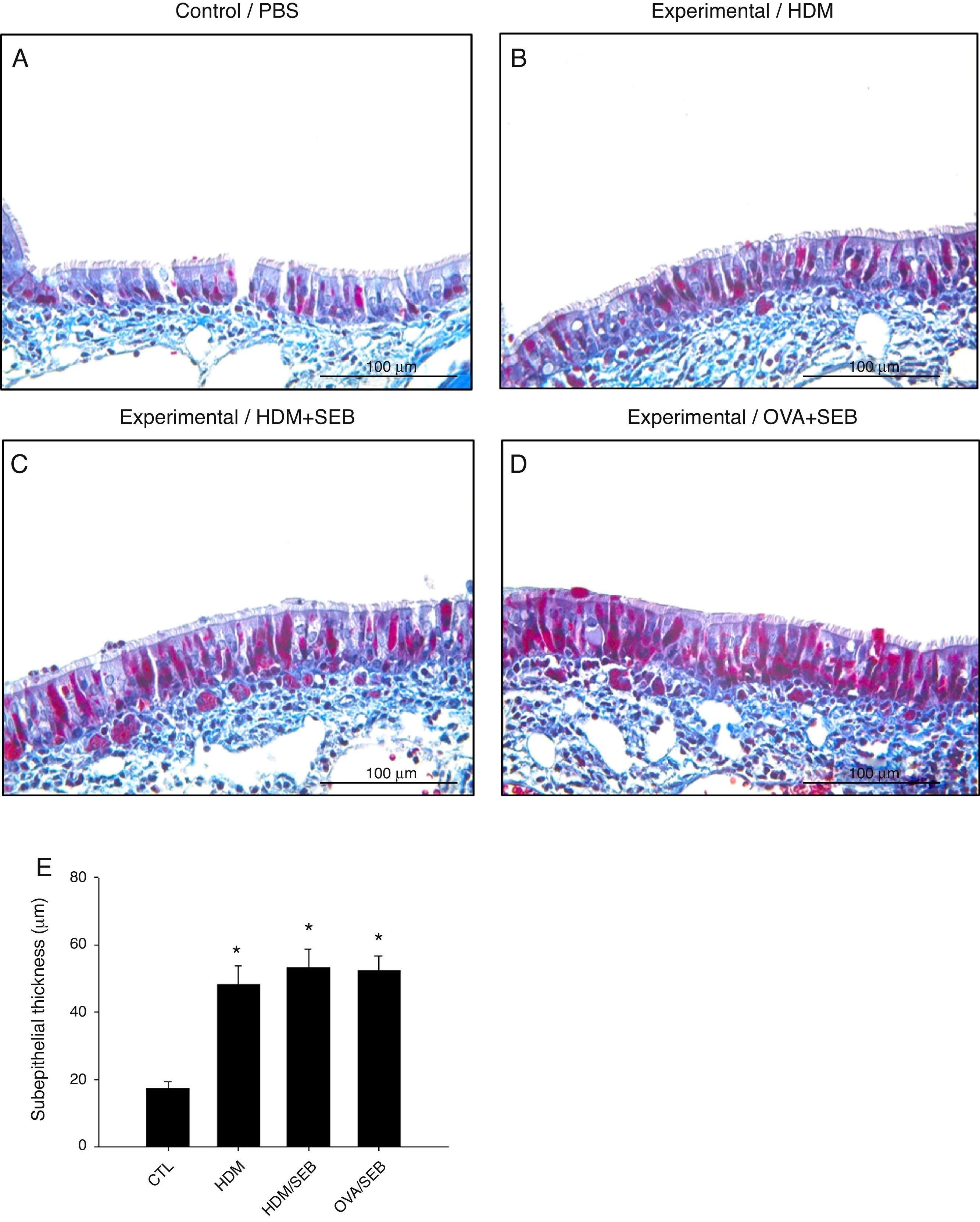
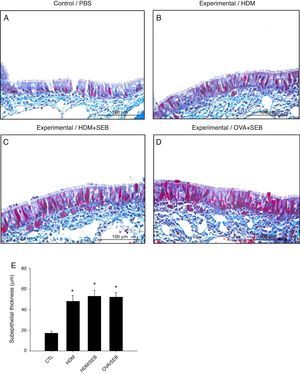
Masson's trichrome staining of nasal mucosa tissue revealed accumulation of collagen in groups B, C and D (Fig. 6). The subepithelial thicknesses (mean±SD) in groups A, B, C and D were 17.3±2.0, 48.2±5.6, 53.2±5.4 and 52.2±4.6μm.
Comparison of subepithelial thicknesses among the groups. (A–E) Masson's trichrome staining showed that groups B, C and D had increased subepithelial collagen deposition compared with control. *p<0.05, compared to group A (CTL); Mann–Whitney U test. Data are expressed as means±SD. Scale bar=100μm.
The inflammatory response to intranasal delivery of HDM was characterised by infiltrate with a considerable proportion comprised of eosinophils and mast cells. Our data demonstrated that mast cells might play an important role in the generation of Th2 sensitisation and airway eosinophilic inflammation in C57BL/6 mice exposed to HDM extract in vivo. Yu and Chen17 demonstrated that Der f could rapidly activate mast cells in mice. Der f could trigger the release of mMCP-1 in mice 30min after an intratracheal challenge, indicating an early activation of airway mucosal mast cells after an encounter with inhaled HDM allergens. In contrast, OVA did not activate mast cells in mice inoculated in a similar manner. Importantly, in Der f-challenged mice, the allergic features were significantly lower or absent after blocking the mast cells using sodium cromoglycate, a mast cell stabiliser. These data indicated that mast cells might be an important cell type during the initiation of Der f sensitisation in the airway.17 Similarly, we found a significantly higher number of mast cells in the nasal mucosa in mice challenged with HDM compared to the OVA/SEB-treated group.
In vivo activation of mast cells appears to be a critical step in the development of HDM-induced allergic inflammation. Upon activation, mast cells release pro-inflammatory mediators such as tryptase, histamine, serotonin, lipid mediators such as PGE2 and LTB4, and a vast range of interleukins.18–20 Mast cells might contribute to changes related to chronic allergy. Mast cells are major effector cells contributing to allergic conditions, but their precise involvement in HDM-induced allergic inflammation is unclear.
Mast cells are involved in the pathophysiological process of CRSwNP.21–23 Interestingly, patients with nasal polyps sensitive to HDMs had higher tryptase, histamine and eosinophil cationic protein (ECP) levels in the nasal lavage fluid than patients without nasal polyps. ECP is a well-described and standardised marker of tissue eosinophilia and activation of eosinophils.24 In the present study, nasal polypoid lesions in mice were characterised mainly by the presence of recruited effector cells—mast cells and eosinophils—typical of allergic inflammation. These results imply that mast cells along with eosinophils play potential roles in the development of nasal polyps.
Although treatment with HDM alone recruited eosinophils and mast cells, inflammation was insufficient to create nasal polypoid lesions in the sinonasal tissue. Administration of low-dose SEB in underlying allergic inflammation induced nasal polypoid lesions with increased eosinophilic infiltration, which corresponds to a previous report.4 Nasal exposure to staphylococcal enterotoxin enhances the development of allergic rhinitis in mice.25 HDM-induced allergic airway inflammation in mice, in combination with SEB, is emerging as a physiologically relevant model for human CRSwNP; this model shares many of the features of human CRSwNP including tissue eosinophilia, goblet cell hyperplasia, epithelial thickening, and subepithelial fibrosis.
In recent years it has become clear that airway epithelial cells, the first cells exposed to inhaled airborne allergens, play a role beyond passive barrier function, as they actively contribute to the induction of the allergic response. In particular, the release of interleukin (IL)-25, IL-33 and thymic stromal lymphopoietin (TSLP) from nasal epithelial cells in response to allergen induces the recruitment and the activation of dendritic cells, mast cells, basophils and eosinophils to promote Th2-biased airway inflammation.26–28 Studies on epithelial-derived cytokines in response to allergen challenge may lead to an understanding of the signalling pathways and the cellular feedback loops involved. Moreover, such studies may shed light on how the immune system senses HDM allergens and triggers aberrant inflammation.29
Advances in the understanding of the pathophysiology of CRSwNP are made possible with animal models. In considering the use of mice, several distinct components need consideration: the allergen, the genetic background of the mice, the experimental approach and the outcome measures. BALB/c and C57BL/6 mice are the most widely used strains due to their well-characterised immunological responses.11,12,30 The dose of antigen used for sensitisation and the route of administration are important variables in the models. Although no mouse model fully mimics the full range of clinical manifestations of CRSwNP, many reproduce a collection of the features that characterise its most common forms.31
The HDM challenge model of CRSwNP may increase our understanding of the basic mechanisms of allergic inflammation and the underlying immunological response, and specific questions can be addressed that are difficult to study in patients. In particular, further studies are planned to focus on the role of cytokines in the chronic airway changes associated with this model. Improved animal models that more closely reflect CRSwNP in relevant human systems will broaden our knowledge of the disease, and might help in the identification and evaluation of new therapeutic targets.
Conflict of interestThe authors have no conflict of interest to declare.
FundingThis work was supported by grant no 03-2014-0120 from the SNUH Research Fund, by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (0411-20130037), by the Education and Research Encouragement Fund of Seoul National University Hospital (2015), and by Research Resettlement Fund for the new faculty of Seoul National University.
Ethical responsibilitiesProtection of human subjects and animals in researchProtection of human and animal subjects. The authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Patients’ data protectionConfidentiality of Data. The authors declare that no patient data appear in this article.
Right to privacy and informed consentRight to privacy and informed consent. The authors declare that no patient data appear in this article.