Urinary leukotriene (LTE4) is an important marker of airway inflammation presence. A relationship between single nucleotide polymorphism in the glucocorticoid receptor (GCR) gene promoter (Bcl I polymorphism), development of asthma and sensitivity to glucocorticoids has been hypothesised.
ObjectiveTo explore the possible association between the Bcl I polymorphism and baseline levels of urinary LTE4 in preschoolers with recurrent wheezing episodes. We prospectively enrolled and classified 86 preschoolers based on the risk of developing asthma (by the Asthma Predictive Index [API]).
MethodsAt admission standardised questionnaires for demographics and respiratory illness characteristics were completed. The Bcl I polymorphism of the GCR was determined by a PCR–RFLP assay from blood samples, and urinary leukotriene was assessed from urine samples by an enzyme immunoassay.
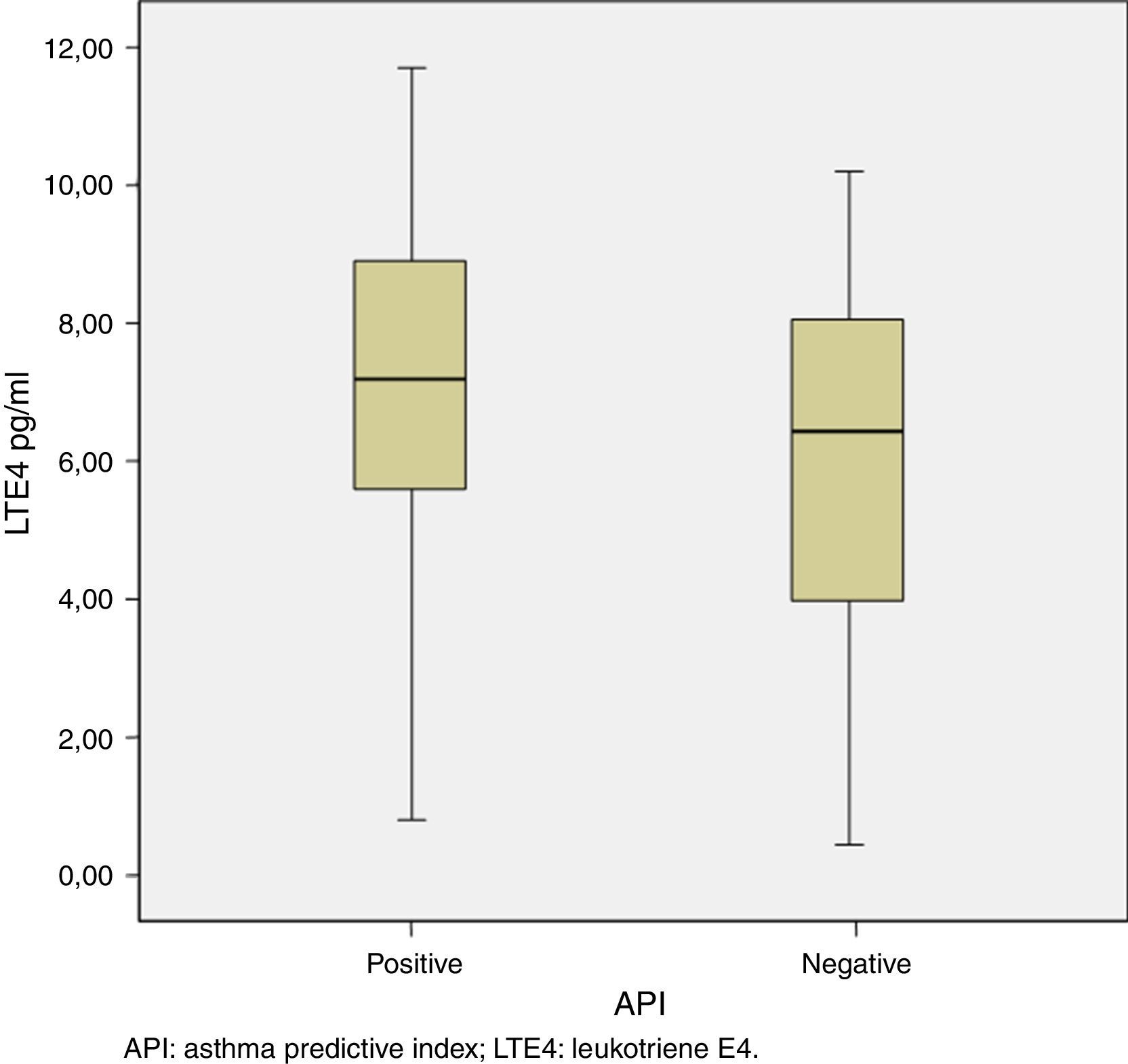
ResultsWe enrolled 86 preschoolers (46 with positive API and 40 with negative API). There were no statistical differences in demographic, respiratory illnesses and wheezing episodes characteristics between both groups. Also, the prevalence of Bcl I polymorphism was similar between positive vs. negative API groups (34.8% vs. 38.9% for homozygote GG, 56.5% vs. 52.8% for heterozygote GC, 8.7% vs. 8.3% for homozygote CC, respectively, p=0.94). However, urinary LTE4 (median [IQR]) was higher in preschoolers with positive than negative API (7.18 [5.57–8.96pg/ml] vs. 6.42 [3.96–8.07pg/ml], p=0.02, respectively).
ConclusionsIn our population, wheezing preschoolers with positive API exhibit higher levels of urinary LTE4 than those with negative API; but there were no differences in Bcl I polymorphism of the GCR.
In the majority of asthma cases, symptoms begin during the first years of life. Recurrent wheezing is an important problem in young children; fortunately, it is estimated that only 30% of preschoolers with recurrent wheezing still have asthma at the age of 6 years.1 However, diagnosis of asthma at this age is difficult, and the use of genetic or biochemical markers for asthma diagnosis remains controversial.2 The risk of developing asthma among those preschoolers with recurrent wheezing can be determined using clinical parameters such as the Asthma Predictive Index (API).3–6
Nevertheless, it has not been clearly determined if those preschoolers with recurrent wheezing and high risk of developing asthma (namely positive API) would have a better response to inhaled corticosteroids (ICS) or to leukotriene receptor antagonist (LTRA) treatment.7–9 An international guideline proposed to start with ICS as a first line of treatment and if there was no response, to change to LTRA.10
Sensitivity to glucocorticoids has been associated with a single nucleotide polymorphism (SNP) within the glucocorticoid receptor (GCR) gene promoter (located on chromosome 5q31-q32, of nine exons). From these, the SNP known as Bcl I polymorphism has been associated with an increased risk of asthma and sensitivity to glucocorticoids.11–13 Patients harbouring the minor allele G versus the C allele develop asthma more frequently, indicating that the presence of the major allele C is associated with a reduced risk of the disease.14
Cysteinyl leukotrienes (LTC4, LTD4, and LTE4) play a role in the chronic inflammatory response of airways.15,16 LTE4, the main metabolite of leukotriene metabolism, is excreted in urine17; and has been used as a marker of airway inflammation presence.18–20 However, urinary concentrations of LTE4 and its association with some clinical parameters have not been fully studied.
The aim of the present study was to determine the possible association between the Bcl I polymorphism of the GCR and the levels of urinary LTE4 in a group of preschoolers with recurrent wheezing episodes (positive or negative API).
Materials and methodsWe performed a case–control study in which patients aged 2–5 years old with recurrent wheezing episodes (≥3 episodes/year diagnosed by physician) and without treatment with LTRA for at least 2 weeks, were sequentially invited to participate in the study. Patients with positive stringent API index (one of two major criteria [eczema or parental asthma] or two of three minor criteria [allergic rhinitis, wheezing without colds or eosinophilia ≥4%]) were considered cases, while patients with negative API were considered controls. Exclusion criteria included patients with chronic respiratory illness (e.g. cystic fibrosis, bronchopulmonary dysplasia, bronchiolitis obliterans, primary ciliary dyskinesia, etc.), cardiopulmonary congenital diseases, gastro-oesophageal reflux or history of any acute respiratory infection in the 3 weeks prior to enrolling.
After explaining the purpose of the study, written informed consent was obtained from parents or guardians of the patient. Data were recorded by a standardised questionnaire and a review of clinical records, including demographic data, age of onset of recurrent wheezing, frequency of episodes in the previous year, severity, parental report of respiratory illnesses (e.g. respiratory syncytial virus (RSV) infection, pneumonia, acute media otitis and croup), anti-asthmatic medication used, visits to emergency department (ED), hospitalisations, family history of asthma/allergic disease and environmental factors (dampness, carpets, pets, tobacco exposure and fuel for heating). At enrolment a total peripheral blood sample for eosinophil and Bcl I polymorphism of the GCR determination was obtained. Samples were frozen at −80°C until processing. The genetic material (DNA) was isolated using a QIAamp DNA Minikit (QIAGEN®, Valencia, CA, US) according to the manufacturer's specifications. Amplification of the DNA segments for the Bcl I polymorphism of the GCR was conducted using a forward primer (5′-CACCAATTCCTCTCTTAAAGAGATG-3′) and reverse primer (5′-AGCAGAAGTACTAACAAAGAGCCC-3′) by polymerase chain reaction–restriction fragment length polymorphism (PCR–RFLP) assay. The amplification was then incubated with a specific restriction enzyme (SfaNI, Fermentas,® Vilnius, Lithuania) for the allele discrimination and samples resolved on acrylamide gel and were visualised by staining with silver nitrate (determination of homozygous or heterozygous allele). Simultaneously, urine samples were collected (by spontaneous urination or by urine collector) for the determination of urinary LTE4 levels. Urine samples were then frozen at −70°C and saved until all samples were processed. LTE4 in urine was measured by ACETM (Enzyme Inmunoassay Kit,® Cayman Chemical, Ann Arbour, MI, USA), according to manufacturer's recommendations. Urinary LTE4 concentration was reported in pg/ml.
The study was approved by the ethical committee of the Pontificia Universidad Católica de Chile (# 11–038).
Statistical analysisA sample size of 41 patients in each group was calculated with a power of 80% and confidence level of 95% for the polymorphism outcome. Comparison of variables between cases (positive API) and controls (negative API) was performed using the chi2 test or Fischer test for categorical variables; and the t-test or Mann–Whitney U-test for continuous variables with or without normal distribution, respectively. Levene test was used for assumptions of normality and equal variances. Continuous variables with normal distribution were described as mean±SD, and those without normal distribution as median and interquartile range [IQR] (25–75 percentiles). p-Values<0.05 were considered to indicate a significant difference. A multivariate logistic regression analysis was performed to determine which variables were associated with positive API condition (dependent variable). Two models were tested: the first model including all the variables as independent, and the second including only those with p value<0.1 in the univariate analysis. Statistical analyses were performed using statistical software (SPSS® v.17, IBM, Armonk, NY, USA).
ResultsEighty-six out of 101 (85%) preschoolers invited agree to participate. We enrolled 86 preschoolers (46 cases and 40 controls). Of all patients, 57% were boys and the age (mean±SD) was 4.01±0.9 years. The age of onset of recurrent wheezing episodes (mean±SD) was 14.9±11.9 months. There were no statistical differences between groups in age, gender, gestational age and birth weight, age of first wheezing episode, ED visits, severe wheezing exacerbations or hospitalisation, use of oral steroids, ICS and salbutamol use, RSV infection and respiratory illnesses (pneumonia, acute media otitis and croup) by parent report (Table 1). No differences in parental education, siblings, day care attendance, type of fuel for heating, dampness, and pets, were found (data not shown).
Demographic and respiratory illnesses characteristics between cases (+API) and controls (−API).
| CasesPositive APIn=46 | ControlsNegative APIn=40 | p-Value | |
|---|---|---|---|
| Males gender | 59.2 | 40.8 | 0.27 |
| Age (years) | 3.87±0.90 | 4.18±0.84 | 0.06 |
| Age of onset wheezing (months) | 14.52±11.52 | 15.41±12.60 | 0.39 |
| Gestational age (weeks) | 38.63±1.80 | 38.11±1.89 | 0.21 |
| Frequency of wheezing in last year | 3.80±2.74 | 4.08±2.86 | 0.65 |
| Severity of wheezing: | 0.56 | ||
| Mild | 34.8 | 46.2 | |
| Moderate | 50.0 | 43.6 | |
| Severe | 15.2 | 10.3 | |
| Seasonality of wheezing: | 0.14 | ||
| Winter | 43.5 | 36.8 | |
| Spring and summer | 13.0 | 2.6 | |
| All seasons | 43.5 | 60.5 | |
| RSV infection | 32.6 | 27.5 | 0.64 |
| Cough with exercise | 80.4 | 60 | 0.056 |
| Inhaled corticosteroids use: | 0.95 | ||
| None | 26.1 | 22.5 | |
| Regular (≥70%) | 58.7 | 62.5 | |
| Irregular (<70%) | 15.2 | 15.0 | |
| Oral corticosteroids use | 69.6 | 57.5 | 0.79 |
| Salbutamol use: | 0.77 | ||
| Occasional (<30%) | 10.9 | 20.0 | |
| Irregular (30–70%) | 41.3 | 42.5 | |
| Regular (≥70%) | 47.8 | 37.5 | |
| ED visit for exacerbation | 64.4 | 66.7 | 0.71 |
| Hospitalisation for exacerbation | 21.7 | 15.0 | 0.34 |
| Tobacco exposure | 48.8 | 51.2 | 0.51 |
| Carpet | 39.1 | 22.5 | 0.11 |
| Respiratory illnesses: | |||
| None | 17.4 | 20.0 | 1.0 |
| Pneumonia | 43.5 | 42.5 | |
| Otitis, croup or sinusitis | 39.1 | 37.5 | |
Numbers are expressed as mean±SD or % when correspond.
API=Asthma Predictive Index; RSV=respiratory syncytial virus; ED=emergency department.
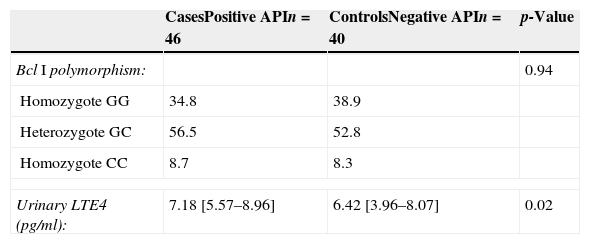
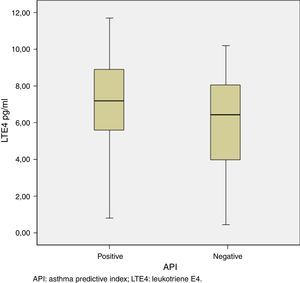
The median [IQR] of urinary LTE4 was significantly higher among preschoolers with positive vs. negative API (7.18 [5.57–8.96pg/ml] vs. 6.42 [3.96–8.07pg/ml], p=0.02, respectively) (Table 2 and Fig. 1). There was no LTE4 undetectable measurement in any of the urine samples. The prevalence of Bcl I polymorphism of glucocorticoid receptor gene was similar between children with positive vs. negative API (34.8% vs. 38.9% for homozygote GG; 56.5% vs. 52.8% for heterozygote GC; and 8.7% vs. 8.3% for homozygote CC, respectively, p=0.94) (Table 2).
Comparison of Bcl I polymorphism of glucocorticoid receptor gene and urinary LTE4 levels between cases (positive API) and controls (negative API).
| CasesPositive APIn=46 | ControlsNegative APIn=40 | p-Value | |
|---|---|---|---|
| Bcl I polymorphism: | 0.94 | ||
| Homozygote GG | 34.8 | 38.9 | |
| Heterozygote GC | 56.5 | 52.8 | |
| Homozygote CC | 8.7 | 8.3 | |
| Urinary LTE4 (pg/ml): | 7.18 [5.57–8.96] | 6.42 [3.96–8.07] | 0.02 |
Numbers are expressed as % or median [IQR] correspondingly.
API: Asthma Predictive Index; LTE4: leukotriene E4.
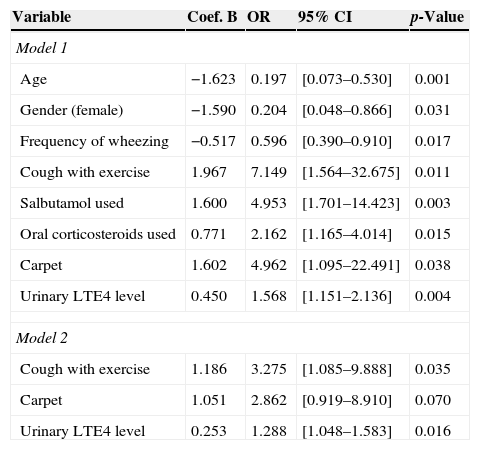
In the first logistic regression model after adjustment for co-variants (including all variables) urinary LTE4 levels still remain significantly associated with positive API (OR=1.56; CI 95% [1.15–2.13], p=0.004). The probability of having a positive API was also associated with older age, males, high frequency of wheezing, presence of carpet, cough with exercise, increased use of salbutamol and oral corticosteroids (Table 3). In a second model (including variables with p-values<0.1 in univariate analysis) the presence of cough with exercise and urinary LTE4 levels remains significantly directly associated with positive API (p=0.03 and p=0.01, respectively) (Table 3).
Multivariate logistic regression for positive API condition.
| Variable | Coef. B | OR | 95% CI | p-Value |
|---|---|---|---|---|
| Model 1 | ||||
| Age | −1.623 | 0.197 | [0.073–0.530] | 0.001 |
| Gender (female) | −1.590 | 0.204 | [0.048–0.866] | 0.031 |
| Frequency of wheezing | −0.517 | 0.596 | [0.390–0.910] | 0.017 |
| Cough with exercise | 1.967 | 7.149 | [1.564–32.675] | 0.011 |
| Salbutamol used | 1.600 | 4.953 | [1.701–14.423] | 0.003 |
| Oral corticosteroids used | 0.771 | 2.162 | [1.165–4.014] | 0.015 |
| Carpet | 1.602 | 4.962 | [1.095–22.491] | 0.038 |
| Urinary LTE4 level | 0.450 | 1.568 | [1.151–2.136] | 0.004 |
| Model 2 | ||||
| Cough with exercise | 1.186 | 3.275 | [1.085–9.888] | 0.035 |
| Carpet | 1.051 | 2.862 | [0.919–8.910] | 0.070 |
| Urinary LTE4 level | 0.253 | 1.288 | [1.048–1.583] | 0.016 |
API: Asthma Predictive Index; LTE4: leukotriene E4.
This study showed a significantly higher urinary LTE4 concentration in preschoolers with positive API compared to those with negative API. No differences in the prevalence of Bcl I polymorphism of the GCR were found between positive and negative API.
Some studies have hypothesised a causal relationship between the presence of polymorphisms of glucocorticoid receptor gene with develop of asthma and sensitivity to glucocorticoids.21 A study on Polish adults found a correlation between Bcl I polymorphism and the occurrence of asthma (e.g. frequency of polymorphism in 70 healthy subjects vs. 59 asthmatics was: 12.9% vs. 43.6% for GG, 47.1% vs. 45.5% for GC, and 40% vs. 10.9% for CC, p=0.00029); meaning that asthmatics had G allele significantly more often.14 In contrast, Szczepankiewicz et al. did not find differences in the distribution of four polymorphisms of GCR gene (including the Bcl I) with the presence of asthma in Polish schoolchildren (n=113 asthmatic and n=123 healthy).13 We did not find differences in the frequency of Bcl I polymorphism of the GCR between preschoolers with positive and negative API; however, since our population was preschoolers (e.g. more heterogeneous wheezing group), a bigger sample would need to confirm this finding and including a healthy group.
We found a significantly higher level of urinary LTE4 among preschoolers with positive API in comparison to those with negative API. In contrast, Kott et al. did not find differences in urinary LTE4 concentrations between infants with history of parental asthma compared to those without parental asthma.18 Another study showed that children with moderate to severe asthma have higher urinary LTE4 levels than healthy controls.22 Unfortunately, we did not have a healthy group to compare their urinary LTE4 levels. Urinary LTE4 levels are elevated in other conditions such tobacco exposure, viral wheeze, RSV bronchiolitis, urticaria, atopic dermatitis, aspirin-induced asthma and exercise-induced asthma.16,18,23–25 In our population no differences were found in the prevalence of tobacco exposure, RSV and respiratory illnesses between positive and negative API groups. Our patients with positive API had more often cough with exercise and most of them had atopic dermatitis as a major criteria of API. Urinary LTE4 levels also increase during exacerbation episodes and decrease to normal ranges during symptoms resolution.16,26 In the present study, in order to not alter the urinary LTE4 levels, the samples were taken with at least 3 weeks free of exacerbations and without LTRA treatment for at least 2 weeks.
It was reported that infants with positive API already have lower lung function.27 Rabinotich et al. found a decrease of 5% in FEV1 per IQR increase in urinary LTE4 level and significant accompanied by increased use of bronchodilator.28 In the present study, the increased use of salbutamol and urinary LTE4 level were independently associated with positive API (model 1). A recent randomised study comparing budesonide vs. montelukast for 24 weeks on preschoolers with recurrent wheezing (n=239) showed that budesonide and montelukast worked equally (improving clinical score) among those with negative API, but montelukast worked better in the positive API group.29 In contrast, a study on preschoolers (n=238) with moderate-to-severe intermittent wheezing, episodic use of either budesonide or montelukast early in respiratory tract illnesses, improved symptoms and activity scores only in children with positive modified API.8 Whether our finding of higher urinary LTE4 concentration in preschoolers with positive API compared to those with negative API means to treat preschoolers (independently of their API condition) with ICS and to use LTRA only for those with positive API needs to be determined in future cross-over trials that include positive and negative API preschoolers.
The present study has some limitations. First, although we reached the sample size for this specific Bcl I polymorphism for the GCR, the number is small and other polymorphisms of GCR gene need to be investigated. Second, even though a statistically significant higher LTE4 levels among preschoolers with positive than negative API was found, these results need to be taken carefully because an overlap exists. Studies with a bigger sample are needed to confirm this finding. Third, no healthy group was included. Fourth, only one sample of urinary LTE4 was measured in these preschoolers without LTRA treatment; therefore, it is possible that obtaining consecutive samples and without ICS treatment would give us a better approach for urinary LTE4 determination. Previous studies showed conflicting results about the effect of LTRA and ICS treatment on urinary LTE4 levels.30,31
In conclusion, preschoolers with recurrent wheezing and positive API have higher levels of urinary LTE4 than those with negative API. However, there were no differences in the prevalence of Bcl I polymorphism in the GCR between groups. Cross-over trials with LTRA and ICS in preschoolers with positive and negative API need to be performed to determine the best choice to treat these populations (ICS or LTRA).
FundingThis study has been sponsored by the Research Grant (#PG 12/11) from Dirección de Investigación Médica, School of Medicine, Pontificia Universidad Católica de Chile, Santiago, Chile.
Conflict of interestsDr. Castro-Rodriguez has participated as a lecturer, advisor, and speaker in scientific meetings and courses under the sponsorship of AstraZeneca, GlaxoSmithKline, Merck Sharp & Dohme, and Novartis. The other authors declare they have no conflict of interests.
Ethical disclosuresConfidentiality of dataThe authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Protection of human subjects and animals in researchThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
What is already known about this topic?
- •
The risk of developing asthma among those preschoolers can be determined using clinical parameters such as the Asthma Predictive Index (API).
- •
The Bcl I polymorphism of glucocorticoid receptor (GCR) gene has been associated with an increased risk of asthma, and urinary LTE4 has been used as a marker of airway inflammation presence.
What does this article add to our knowledge?
- •
Preschoolers wheezing with positive API exhibit higher levels of urinary LTE4 than those with negative API; but there were no differences in Bcl I polymorphism of the GCR.
How does this study impact current management guidelines?
- •
Cross-over trials with LTRA and ICS in preschoolers with positive and negative API need to be performed to determine the best choice to treat these populations.
We thank Mr. Oslando Padilla Pérez, MSc. for statistical support, and Mr. Anthony Carlson for his English editorial assistance.
The data was partially presented in the ATS Annual Congress, San Diego, CA, 2014.