INTRODUCTION
Colostrum and milk are important secretions with nutritional and immunological properties. Many infections could be prevented by encouraging mothers to breast-feed their newborns (1, 2).
Diarrhea represents one of the major causes of morbidity and mortality among infants of low socio-economic levels in developing countries. Enteropathogenic Escherichia coli (EPEC) is an important cause of acute diarrhea in the first year of life in such countries (3). In São Paulo, Brazil, EPEC is the most frequently detected pathogen in children under 12 months of age with acute diarrhea, and the serogroups O111 and O119 are most frequently isolated (4, 5).
Methods for assessing bacterial adhesion include assays performed with HeLa or HEp-2 cells in culture. In such systems, EPEC strains show a localized adherence (LA) specific pattern (6). Studies by Cravioto et al (7) and Silva and Giampaglia (8) showed that this system is suitable to study the inhibitory efect of human milk and colostrum on the adherence of EPEC to cultured cells.
Regardless of serogroups commonly found as EPEC, virulence is determined by several factors including the presence of a 50 to 70 Mda plasmid, EAF (EPEC adherence factor) and a chromosomal pathogenicity island, LEE (locus of enterocyte effacement), which encodes a type III secretory apparatus (9).
A four-stage model of EPEC interaction with epithelial cells was recently proposed by Knutton et al (10). According to this model, growth of bacteria in tissue culture results in the expression of bundle-forming pili (BFP) encoded by EAF plasmid (11); expression of intimin, a 94-kDa outer membrane protein; and production of EspA (EPEC secreted proteins) filaments (stage 1). BFP and EspA form a direct link between the bacterium and the host cell, and among bacteria themselves, resulting in adhesion of bacteria in a localized pattern (6). Secreted proteins are translocated into host cell, including EspB and Tir (translocated intimin receptor), and probably other effector proteins yet to be identified. This in turn leads to tyrosine protein kinase (TPK) activation and display of the intimin receptor, which is a LEE-encoded bacterial protein (tyrosine phosphorilated Tir), and to actin rearrangements (stage 2). Intimin brinds to phosphorylated Tir, and polymerized actin accumulates beneath intimately attached bacteria (stage 3). Further actin polymerization produces mature attaching and efficacing (A/E) lesion (12). At this stage, all EspA filaments and intimin have been eliminated from the bacterial surface (stage 4). This stage comprises loss of host cell microvilli, formation of pedestals beneath the adherent bacteria, and rearrangements of host cell cytoskeleton.
Colostrum and human milk protect the neonate intestinal mucosa against EPEC infections by inhibiting bacterial adhesion to epithelial cells (13). This effect has been shown to be mediated by colostrum and milk SIgA (secretory immunoglobulin A) (14, 15), and by oligossaccharides present in human milk (7).
There are clinical conditions in which breast-feeding is not possible, including maternal diseases and newborn immaturity or physical disorders. A protective effect of bovine milk immunoglobulins has been shown in infants with diarrhea caused by rotavirus (16) and EPEC (17), and in HIV + adults infected with Cryptosporidium (18).
The aims of the present study were to investigate the effects of colostrum and milk from cows vaccinated with EPEC on the adherence of the EPEC O111 strain to HEp-2 cells, and to investigate the milk component involved in these effects. Bovine colostrum or milk, if effective in providing inhibition of bacterial adherence, could be useful sources of antibodies to be used in the absence of natural breastfeeding, in high-risk nurseries, or in preventing nosocomial infections.
MATERIALS AND METHODS
Immunization of cows
Four pregnant Friesian cows (numbered from 1 to 4) in a dairy farm in São Paulo, were immunized 7 to 9 times during the last 10 weeks of gestation. Each cow was injected subcutaneously with 1 ml of a suspension of inactivated EPEC strains of serogroups O111, O119 and O55 (107-108 bacteria/ml), in 0.5 % formol in saline solution with the adjuvant Avridine (Pfizer). All bacterial strains were AL+, BFP+. Four non-immunized cows (numbered I to IV), and a pool of human colostrum were used as controls. Blood samples for determination of anti-EPEC antibodies were obtained from the cows prior to immunization and after the last injection.
Preparation of colostrum and milk
Colostrum from the immunized and control cows was obtained within the first 24-48 h post-partum. Subsequent milking was performed after 6 to 16 days (sample A), 21 to 32 days (sample B) and one year (sample C, from cow #2) post-partum. Colostrum and milk samples were delipidated and stored at 20 °C until assayed.
Studies on industrialized milk
The following formulas were analyzed: skim milk Molico (five cans from different lots), NAN 1, NAN HA and Alfaré (both depleted of proteins by enzymatic treatment) and soy based formula Alsoy (all from Nestlé Industrial e Comercial Ltda., São Paulo).
Industrialized milk components, including potassium caseinate (6.4 g/l), lactose (74 g/l), soy lecithin (1.3 g/l) and maltose (22.3 and 74 g/l) diluted in 1 % solution of Tween, or in PBS, were also analyzed.
Fractionation of colostrum and milk samples
Fifteen ml aliquots of colostrum samples from three immunized animals (cows #1, 3, and 4), and one non-immunized animal (II); two samples of Molico (lots #2 and 5) and milk formulas NAN 1, NAN HA, Alfaré and Alsoy, were fractionated into low (LMWF) and high molecular weight fractions (HMWF), using an Amicon Centriprep (Amicon, Inc., Beverly, MA, USA), with a molecular weight cut off of 10 kDa. HMWF were reconstituted to the original volume after fractionation. Absorption of cow #1 HMWF with EPEC strain O111:H-HMJ 0041-1-85 (AL+, BFP+) was performed as previously described (14).
IgG purification on affinity column
Six ml aliquots of colostrum from cows #1 and #II were depleted of IgG in a protein G-Sepharose column. Subsequent elution of IgG from the column was performed using glycine-HCl pH 2.8. The initial volume of all materials was restored after affinity chromatography to maintain the original concentration.
Adhesion assays
The EPEC strain O111:H, HMJ 0041-1-85 (LA+, eaeA+, EAF+,bfp+), isolated from the stools of a child with acute diarrhea from São Paulo, Brazil, was used for adhesion assays. There were carried out as described by Silva and Giampaglia (8). Briefly, HEp-2 cells (ATCC-CCL 23) were grown in Lab-Tek chamber slides (Nunc Inc., Denmark), added by a mixture of colostrum or milk and a standardized bacterial suspension in medium with 2 % fetal calf serum and 10 % D-mannose to prevent non-specific adhesion. After incubation for 30 minutes, unattached bacteria were removed by washings, and a new medium without colostrum or milk was added, followed by incubation for 3 h. Cells were then washed, fixed with methanol, and stained with May-Grünwald and Giemsa. At least 300 HEp-2 cells were observed in each preparation under light microscope (*400 or *1,000). Results were expressed as the percentage of cells with six or more attached bacteria comparing to control cells treated without colostrum or milk. Observation of control cells evidenced that 100 % of HEp-2 cells showed bacterial clusters on their surface. Colostrum and milk had no bactericidal or bacteriostatic effect in bacterial growth in the absence of HEp-2 cells (data not shown).
Antibody determination
Levels of IgG anti-EPEC antibodies in colostrum and milk samples were evaluated by enzyme-linked immunosorbent assay (ELISA), using a method described in Carbonare et al (19). Microtiter ELISA plates (Costar, Cambridge, MA) were coated with a standardized bacterial suspension of E. coli O111. The antigen-antibody reaction was carried out overnight with appropriate dilutions of human and bovine colostrum, bovine formula milk, and all HMWF and LMWF, and revealed by peroxidase conjugated anti-bovine IgG (Sigma, St. Louis, USA) and o-phenylenediamine dihydrochloride (OPD) (Sigma) substrate. Antibody titer was expressed as the dilution necessary for each sample to reach the arbitrary absorbance value of 0.2.
Immunoblotting
Immunoblotting was used to detect antibodies to EPEC antigens, as described by Towbin et al (20) with modifications. Bacterial components were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). After transferring to nitrocellulose membranes, strips were incubated with colostrum or milk samples and revealed with peroxidase conjugated anti-bovine IgG or IgA and 3,3'-diaminobenzidine (DAB) (Sigma) as substrate. Molecular weight standards (Pharmacia, Uppsala, Sweden) were run in each gel.
Statistics
Statistical analysis was carried out using variance analysis with one factor followed by multiple comparison by Tukey method or paired Student t-test. Both were performed with 95 % confidence limits and a probability value p < 0.05 was considered significant.
RESULTS
Effect of cow colostrum and milk on the adherence of EPEC to HEp-2 cells
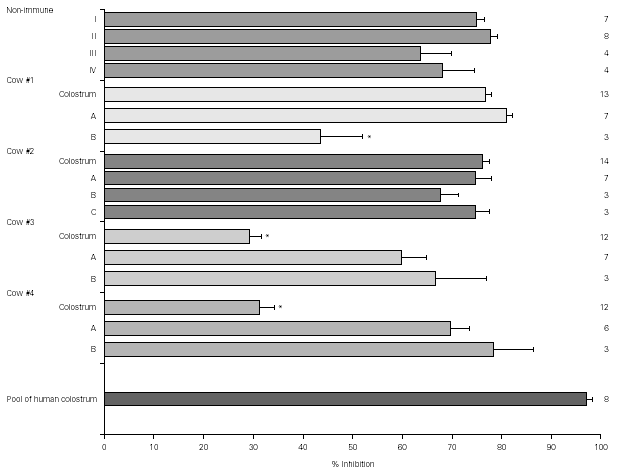
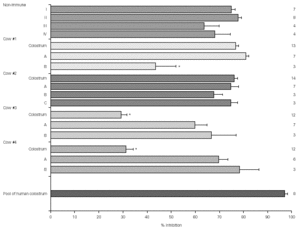
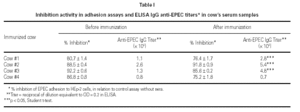
Figure 1 shows the result of EPEC adherence tests in the presence of colostrum samples from non-immunized cows (#I, II, III, IV) and colostrum and milk samples (A, B, and C) from immunized cows (#1, 2, 3, and 4). Tests performed with the pool of human colostrum are shown in the bottom. The effect of colostrum from non-immunized cows on the adherence of EPEC to HEp-2 cells was similar to that of colostrum from immunized animals #1 and #2 and pool of human colostrum. The effect of colostrum from immunized animals #3 and #4 were significantly lower than colostrum from other immunized cows. All milk samples but sample B from cow #1 had high inhibitors levels. The percentage inhibition of EPEC adherence varied greatly among different materials, but all levels were slightly lower than those observed with the pool of human colostrum.
Figure 1.--Resuts of EPEC adherence assays to HEp-2 cells with colostrum from non-immunized cows (#I, II, III, IV) and colostrum and milk samples (A, B, and C) from immunized cows (#1, 2, 3, and 4). Tests performed with the pool of human colostrum are shown in the bottom. All inhibition levels are represented as mean ± standard error. Number of experiments are shown at right. *p < 0.05 by Tukey method.
Effect of cow serum on the adherence of EPEC to the HEp-2 cells
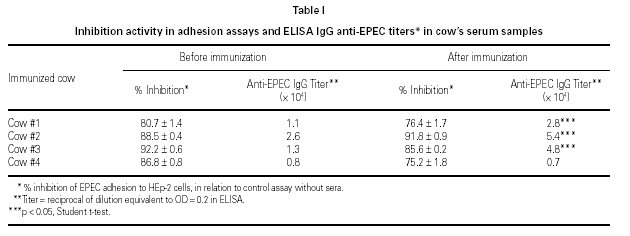
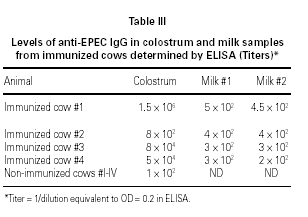
All serum samples presented high degrees of inhibition of bacterial adherence. No significant differences were observed in adherence assay results between cows' serum samples collected before and after immunization with EPEC (t student test). However, post-immunization anti-EPEC IgG titers were higher than pre-immunization ones in 3 of 4 animals (table I).
Effect of milk formulas and milk components on the adherence of EPEC to the HEp-2 cells
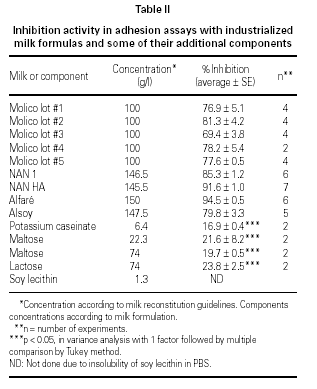
At least 69 % of inhibition of EPEC adherence was observed when milk formulas were used in the assays (table II). This effect was observed when bovine milk formulas Molico, NAN 1, NAN HA, and Alfaré were used, as well as when the soy-based formula Alsoy was used. Milk components (maltose 22.3 g/l and 74 g/l; lactose 74 g/l; and potassium caseinate 6.4 g/l) showed low levels of adherence inhibition, ranging from 17 to 24 %. Results with soy lecithin as well as all other components diluted in 1 % Tween 80 were not considered due to the drastic effect of this detergent upon the bacterial adherence. Differences between inhibition of milk formulas group and milk components group were statistically significant when compared by the Tukey method.
Effect of colostrum and milk formulas LMWF and HMWF on the adherence of EPEC to the HEp-2 cells
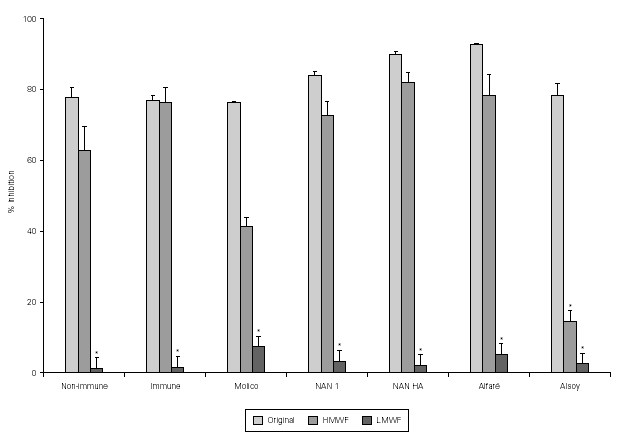
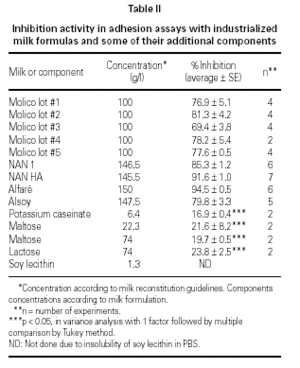
Results of the fractionation experiments revealed that the component responsible for the inhibition of bacterial adherence was present in the HMWF, of both colostrum from immunized and non-immunized animals, NAN 1, NAN HA, and Alfaré. In the cases of Molico and Alsoy, HMWF showed significantly lower values of adhesion inhibition compared to original formulas. HMWF of Molico showed significantly higher value compared to its LMWF, while for Alsoy HMWF and LMWF had equally low values (figure 2). The LMWF had little effect in the assays in all cases.
Figure 2.--Effect of original colostrum, HMWF and LMWF of colostrum samples from one non-immunized animal, one immunized animal, and industrialized formulas (Molico, NAN 1, NAN HA and Alfaré), on EPEC adherence to HEp-2 cells. There were performed 3-4 experiments with each material. *p < 0.05 by Tukey method.
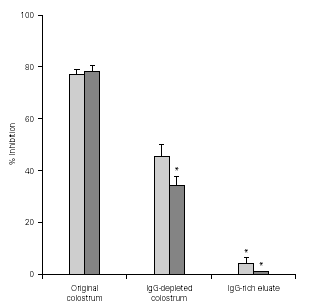
Effect of original colostrum, IgG-depleted colostrum, and IgG-enriched eluate from immunized (#1) and non-immunized cow (#II) on the adherence of EPEC to HEp-2 cells
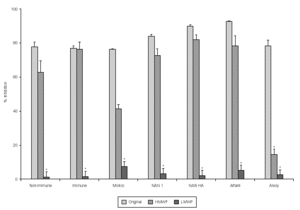
Figure 3 shows that the levels of adherence inhibition by original colostrum, IgG-depleted colostrum, and IgG-enriched eluate from immunized cows were similar to those from non-immunized animals. Greater inhibitory levels were observed with original colostrum. IgG depletion caused a significantly loss in inhibitory activity compared to original colostrum. On the other hand, results using the IgG-enriched eluate showed less than 5 % inhibition of adherence.
Figure 3.--Levels of inhibition of EPEC adherence to HEp-2 cells observed with original colostrum, IgG-depleted colostrum, and IgG-enriched eluate, from one immunized and one non-immunized animal. *p < 0.05 by Tukey method.
Effect of LA+ EPEC-absorbed HMWF on bacterial adherence to HEp-2 cells
Absorption of HMWF with LA+ EPEC strain did not prevent inhibitory effect on bacterial adhesion: original colostrum, 78 % inhibition; HMWF undiluted and diluted 1:2, 77 % and 81 % inhibition, respectively; HMWF pre-absorbed with LA+ EPEC strain, 91 % inhibition.
Antibody determination
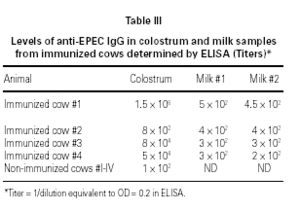
ELISA results showed higher titers of anti-EPEC IgG in colostrum as compared to milk, in 3 o 4 animals. Samples obtained from cow #2 showed some protein denaturation (table III). High molecular weight fractions (HMWF) from immunized animals contained titers of anti-EPEC antibodies equivalent to the respective original colostrum, whereas all LMWF showed negative results (data not shown). Colostrum samples from non-immunized animals showed very low titers of anti-EPEC antibodies < 100.
Immunoblotting
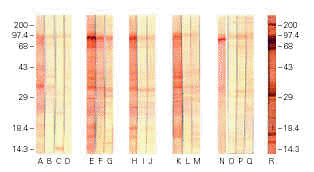
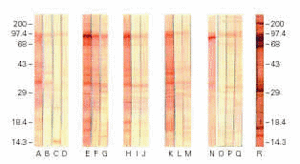
Immunoblotting assays with colostrum from immunized animals and developed with anti-bovine IgG showed strong reactivity with several EPEC antigenic fractions, including the 94 kDa adhesin Intimin, whereas milk from immunized animals and colostrum samples from non-immunized animals exhibited weaker reactions (figure 4). The pool of human colostrum, developed with anti-human IgA conjugate, recognized several EPEC antigenic fractions, as expected. Immunoblotting assays of bovine colostrum samples developed with anti-bovine IgA did not show any reaction (data not shown). Negative reactions were also obtained with industrialized milk and all the LMWFs.
Figure 4.--Immunoblotting of extract of Escherichia coli O111 reacted with samples of non-immunized bovine colostrum (lanes A, B, C, and D), and samples of bovine colostrum and milk of animals #1 (E, F, and G); #3 (H, I, and J); #4 (K, L, and M); 2# (N, O, P, and Q); and human colostrum pool (R).
DISCUSSION
EPEC can cause severe aqueous diarrhea by attaching to human enterocytes, inducing the effacement of their brush border (12). The in vitro model utilized in the present study simulates the interactions between EPEC and ephitelial cells. Using this model, we showed that bovine colostrum and milk samples strongly inhibit EPEC adhesion to HEp-2 cells.
Both IgA anti-EPEC 94-kDa outer-membrane protein (7, 14, 15) and oligosaccharides (7) present in human breast milk are capable of inhibiting EPEC adherence to HeLa and HEp-2 cells. The inhibition of bacterial adhesion or invasion of enterocytes is considered one for the protective effects of human milk against gastrointestinal infections in infants (13).
Bovine milk with some of the protective properties found in human milk would be very useful in certain situations, such as patients with immunodeficiencies, bottle-fed infants and in the prevention of nosocomial gastrointestinal infections in nurseries. In a study in which an immunoglobulin preparation obtained from bovine milk from vaccinated animals was given to children with EPEC diarrhea, 84.3 % of the stool cultures became negative (17). Similar results were obtained in 19 of 25 children with rotavirus gastroenteritis (16).
The mechanism involved in the inhibition of EPEC adhesion to HEp-2 cells by bovine colostrum and milk must be somewhat different from that accepted for inhibition by human colostrum, because the inhibitory activity occurred irrespective of the presence of anti-EPEC antibodies in bovine colostrum or milk.
In the present study, anti-EPEC IgG antibody levels detected by ELISA were higher in colostrum or milk from immunized animals than from non-immunized animals. However, both samples were able to inhibit EPEC adhesion to HEp-2 cell, suggesting that this ability is not related to anti-EPEC IgG antibody titers. Our results are in agreement with those by Rump et al (18). These authors investigate the effect of treating immunodeficient patients with Lactobin-R, an immunoglobulin concentrate obtained from non-immunized cows' colostrum. They showed remission of diarrhea in 51.3 % of the patients after 4 weeks of treatment.
Immunoblotting assays confirmed the presence of specific anti-EPEC antibodies in colostrum samples from immunized animals, including antibodies to a 94 kDa band consistent with Intimin. Weaker bands were detected using samples of milk and colostrum from non-immunized animals.
Serum samples obtained before and after immunization showed an increase in antibody titers detected by ELISA, however there were no changes in the inhibitory levels assessed by adhesion assays. These results confirm that this ability is not related to anti-EPEC antibodies.
Since we observed inhibitory activity in colostrum from non-immunized animals, we performed adhesion assays using industrialized milk formulas which are frequently given to infants in Brazil. All formulas strongly inhibited EPEC adhesion to HEp-2 cells, in the absence of EPEC antibodies, as shown by ELISA or Immunoblotting.
Soluble oligossacharides present in human milk could be responsible for the inhibition of bacterial adherence, by binding to cellular receptors, or preventing the interactions of fimbrial adhesins to human cells (21, 22). Fractionation on bovine colostrum samples to HMWF and LMWF showed that the active component is present in the HMWF, and this is not related to free oligossaccharides, as previously shown for human milk in other systems (7). This is keeping with the observation that bovine milk has a lower content of oligossaccharides, as compared to human milk (23). The presence of anti-EPEC antibodies in HMWF and their absence in LMWF, was confirmed by ELISA and Immunoblotting assays.
Industrialized milk formulas were also submitted to fractionation, and the inhibitory activity remained in HMWF. Adhession assays performed with isolated components of formulas (maltose; lactose; potassium caseinate and soy lecithin) showed that Tween, used in the solubilization of soy lecithin, is probably the active component of milk formulas causing the inhibition of adhesion of EPEC to HEp-2 cells. This observation is in accordance to the fact that linoleic acid, one of the Tween's components, is able to inhibit EPEC adhesion to HEp-2 cells (24).
The next step in the search for the active component that inhibited bacterial adhesion was the isolation of IgG the main immunoglobulin in bovine colostrum, through affinity chromatography in protein G-Sepharose. With regard to this, Doyle et al (25) obtained an immunoglobulin concentrate (55 % of IgG) from bovine colostrum after immunization with Cryptosporidium. In vitro assay showed a 61 % reduction of intracellular parasites by an IgG solution of 100 to 1,000 mg/ml, and no reduction with IgG concentrations below 50 mg/ml.
After isolation through affinity chromatography, IgG enriched eluate from both immunized and non-immunized cows didn't show significant inhibitory activity. In both cases, IgG depleted colostrum showed 50 % reduction of inhibitory activity in relation to the non-fractionated material, suggesting that the fractionation impaired the activity or the loss of IgG could reduce the inhibitory capacity of the whole colostrum. The inability of isolated IgG in inhibiting bacterial adherence could be explained by the impairment of its biological activity by the low pH of the elution buffer. Alternatively this result suggest that IgG may not be responsible for the inhibitory phenomenon.
Data presented here show that cows' immunization with EPEC did not change significantly the degree of inhibition of EPEC adhesion, by neither serum nor colostrum and milk. No correlation was found between inhibitory activity and the presence of anti-EPEC antibodies or IgG in all material studied. IgG antibodies isolated from bovine colostrum did not inhibit EPEC adhesion to HEp-2 cells, whereas IgG depleted colostrum or a fraction of colostrum greater than 10 kDa (HMWF) pre-absorbed with EPEC maintained the inhibitory activity.
In conclusion, we have shown that IgG is not the only active component of bovine milk that inhibits bacterial adhesion to HEp-2 cells. Taken toghether, these results indicate that there may be one or more factors other than immunoglobulin involved in inhibition of EPEC adhesion to HEp-2 cells.
ACKNOWLEDGEMENTS
The authors thank FAPESP for financial support, Nestlé Industrial e Comercial Ltda., for the cows immunization and the Obstetrics Clinic of University Hospital, from the human colostrum samples.