INTRODUCTION
Cupressaceae allergy appears to be a pollinosis of increasing importance in the mediterranean area (1-9). Symptoms begin in january and ends in late march. The increase of respiratory allergy to Cupressaceae pollen (Juniperus oxycedrus, Cupressus arizonica, Cupressus sempervirens) in the mediterranean area ia a well known phenomenon (2). This increase has been the object of many recent publications in Italy: a polycentric study has shown a prevalence of 18 % of Cupressaceae pollen allergy among all the polinosis (11). In relation to this increase, the span of time of the pollinisation season of the different species of Cupressaceae has extended from november to april.
Due to the difficulty of obtaining good extracts of Cupressus sempervirens, which is the main offender in our area, and due to the fact that we have demonstrated, since the beginning of our study, an intensive crossreactivity between the different Cupressaceae and even the Taxodiaceae (Cryptomeria japonica), we have selected the pollen of Cupressus arizonica which is easier to prepare and is chimically stable specially in solution. We have already treated with good results the allergy to Cupressaceae using a mixture of Cupressus sempervirens and Cryptomeria japonica (10). This study has shown the efficacy of SIT in this pathology. We wanted to confirm the efficacy and security of the SLIT of an extract of Cupressus arizonica to treat patients mostly allergic to Cupressus sempervirens.
Classical specific immunotherapy for Cupressaceae pollen allergy has been already used for years by different authors (10, 12-16). Specific immunotherapy (SIT) for the treatment of allergic diseases using, instead of the classical subcutaneous technique, new routes i.e. oral, sublingual and local nasal has already been used since 1980 by different investigators using other allergens: house dust mites, grass, olive, parietaria, birch pollen (17-34). Its efficacy has been generally established by double blind studies; on the basis of these studies the Who Position Paper on allergen SIT, the sublingual specific immunotherapy (SLIT) has been accepted.
We have some experience of specific immunotherapy (SIT) for this pollinosis with the clasical subcutaneous immunotherapy (4, 10). Several factors prompted us to find an alternative to the classical subcutaneous SIT to treat our patients having a respiratory allergy to the pollen of Cupressaceae.
PATIENTS AND METHODS
Patients: in january 1999, 20 patients were selected on the basis of clinical history (hivernal pollinosis, positive skin tests, positive RAST, positive Nasal Provocation test [NTP]) (table I).
They were divided into two matched groups of 10. Males: 9, Females: 11, Average age: 34.8 (s.d. 13.12).
Symptom and drug consumption scores
Patients symptoms were assessed daily using a self-scoring symptom card (table II). Each patients indicated how often attacks of rhinitis occurred, how long they lasted, whether any dyspneal or conjunctival attack appeared and how many drugs were taken during the week. The severity of symptoms were scored as follows: no symptoms = 0; slight symptoms = 1; moderate symptoms = 2; severe symptoms = 3. Drug consumption was also scored: the score was 1 for one administration, and 2 or more for other administration of local systemic drugs. Patients were also clinically examined every two weeks. Side effects: each patient was instructed to note on his diary were the daily dose was written untoward symptoms such as pruritus or burning in the month, nausaea, vomitus, diarrhea or abdominal pain, rhinitis or others. SLIT was initiated in april 1999 and a control was done in may 2000.
Skin testing
Prick tests was performed with the Dome Hollister lancet. Extracts were kindly provided by Anallergo manufacturere (Firenze, Italy) and were use in a 1/20 solution w/vol). The extract was standardizated according to the two associated methods: RAST inhibition and histamine equivalency. We adopted the following scores: a 4+ reaction corresponds to a greater reaction than to histamine. A 3+ reaction corresponds to the wheal and erythema of histamine (1 mg for 1 ml prick test). A 2+ reaction is 75 % and 1+ reaction is half the size of the histamine reaction.
IgE RAST
The RAST was performed as described by Wide (35). In vitro diagnosis for Cupressus sempervirens specific IgE was completed using commercial kits according to the manufacturer instructions (Pharmacia A B, Uppsala, Sweden). We followed the Phadebas RAST UNITS (P.R.U.) system. We used the same extracts as the one used for prick test. A measure of specific IgE to Cupressus sempervirens was carried out at the beginning and at the end of the treatment.
Nasal provocation test
Specific nasal provocation test was carried out period in all patients before and after the study with Cupressus arizonica extract obtained from the manufacturer (Anallergo srl, Firenze, Italy). The extracts were at different concentration: 2.2, 6.6, 20 µg/ml. The patients had to be symptom-free. Tests were performed with the instillation by pression into one nostril of increasing concentration at intervals of 15 minutes. A control test with the dilution buffer was performed into the opposite site. We utilized the Youlten peak inspiratory flow meter (PIFRn meter) (36). It offers some advantage over the expiratory flow rate meter especially when used in provocation tests and it is easier to handle compared with standard rhinomanometry. The results are expressed as the mean values of three consecutive registrations. A test was considered as positive when we observed a drop in the basic value of 20 % and/or when it elicits at least two of the following symptoms (itching, sneezing, rhinorrhea, obstruction).
Pollen counts
The pollen counts of Cupressaceae pollen, for the relevant area, were made with a continuous volumetric apparatus (Hirst spore trap VPPS 2000, Lanzoni srl, Italy) placed 20 m above ground level, far from sources of pollution and exposed to winds. The pollen counts were performed by the personel of the Allergology Department of Bordighera Hospital. Sampling and counting method were those recommended from Italian Aerobiologic Association (A.I.A.) (37, 38). Figure 1 shows the means monthly pollen/m3 during the two following blooming season 1998-1999, 1999-2000.
Figure 1.--Cupressaceae pollen counts. Mean monthly pollen counts grains/m3 from october to april during the 1999 and 2000 Cupressaceae pollen seasons.
Protocol of traitment
The active traitment consisted in an aqueous solution of an allergic fraction of Cupressus arizonica partially purified through dialysis in a physiological solution with 15 % glycerin. As previously stated, the extract was standardizated according to the following associated methods: RAST inhibition and Histamine equivalency. The titration was carried out in RAST UNIT/ml.
The patients were instructed to keep the liquid for at least two minutes under the tongue before swallowing it (SLIT-SWALLOW technique). Schema of treatment: there was 5 vials with the following concentrations (vial n.º 1: 100 U RAST/ml; vial n.º 2: 300 U RAST/ml; vial n.º 3: 1,000 U RAST/ml; vial n.º 4: 4,000 U RAST/ml; vial n.º 5: 10,000 U RAST/ml). The initial phase which lasted 50 days consisted in taking 5 drops every day from vial 1 to vial 5. During the maintenance treatment which lasted 6 months, patients have to take drops of vial 5 every other day. The total average cumulative dosis was 250,000 U RAST for each patient which is five times more than the usual dosage in classical subcutaneous immunotherapy.
Statistical analysis
Non parametric data (score symptoms and nasal provocation tests) were analysed by the Mann-Whitney U test for intergroup comparison and Wilcoxon's rank sum test for intragroup comparison. Normally distributed quantitative variables were compared with Student's tests. All tests were two-tailed and p < 0.05 was considered to be significant.
RESULTS
The results of the efficacy of SLIT with an extract of C. arizonica was assessed according to the 3 following criteria: 1) Symptom scores; 2) Drug score; 3) Nasal provocation Test (N.P.T.).
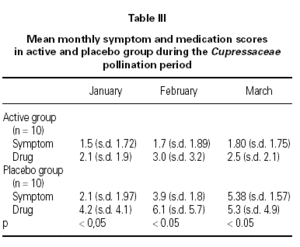
At the beginning of the study the two groups, active and placebo, were comparable concerning age, gender and duration of symptom (table III). During the Cupressaceae pollen season the symptoms scores for conjunctivitis, rhinitis and asthma were significant lower (p < 0.05) in the active treatment group (fig. 2). Also drug consumption was significant less (p < 0.05) in during the whole survey (fig. 3).
Figure 2.--Symptom scores in relation ti pollen acounts. Mean monthly symptom scores in active and placebo group during two consecutive pollen season (before and after SLIT).
Figure 3.--Drug scores in relation to pollen acounts. Mean monthly drug scores in active and placebo group during two consecutive pollen season (before and after SLIT).
The figure 4 shows the changes of threshold dose to Nasal Provocation Test in the active and placebo group. After one year of treatment a significant increase (p < 0.01 by Wilcoxon test) in threshold dose to specific nasal challenge in comparison with baseline values was observed in the active group but not in the placebo group (p < 0.01, Mann-Whitney U-test) (table IV).
Figure 4.--Slit Cupressaceae: nasal challenge. Changes in threshold dose to specific nasal challenge after one year of Cupressaceae pollen SLIT in the active and placebo groups.
According to these results at the end of the study, the active group could tolerate about four times more allergen dose than at the start of the study. A significantly (p < 0.05 chi-square test) greater proportion on the active compared to the placebo group was able to tolerate the highest concentration (table V).
Side effects: all patients completed the study and no local systemic side effects were reported by either group.
DISCUSSION
Since we do not know yet all the allergens major and minor of Cupressus sempervirens but we do know that the extract of Cupressus arizonica is easier to prepare and has a good stability in solution and that there is a broad cross-reactivity between Cupressus sempervirens and Cupressus arizonica pollen, we think justified to use an extract of Cupressus arizonica in Cupressus sempervirens allergy. We found in litterature 18 double-blind studies concerning the efficacy of SLIT with only two negative reports in which an extract of Dermatophagoides pteronyssimus was used. Though a few papers have been published by ourselves and others on the SIT in Cupressaceae allergy using the classical subcutaneous route, there was no report on the use of SLIT. By the time being there is no precise rule of the definition of the optimal and total dose of extract necessary and sufficient to obtain a good result. It seems however, according to our data and the data obtained from litterature; that low doses fare better than high doses.
We shall not discuss here the mechanism of action of this local form of SIT which as been done elsewhere (31), let us just say that there is in litterature a clear evidence of the absorption of the allergens and the immunologiccal effects of this absorption. Besides there is no notable difference between the pollen counts of year 1998-1999 and 1999-2000. It is clear from our results that the treated group fared better than the placebo group according to the three selected criteria: we found a statistically significant difference between the treated group and the placebo as far the clinical symptomatology is concerned (matched age, gender, etc.). The symptom and the drug consumption scores were, after treatment, lower in the treated group but the threshold of allergen reactivity in the N.P.T. was much higher in the treated group. In contrast there were no difference as far as the intensity of skin test and the value of the specific RAST was concerned.
There was no more minor side effects in the treated group than among the placebo group. At any rate we were never obliged to stop the treatment. Of course, it was not possible to compare the results obtained with our technique with, on the one hand, the results using the classical subcutaneous route and on the other hand, the results obtained using an extract of Cupressus sempervirens or another member of the Cupressaceae family such as Juniperus ashei for instance which is also easier to prepare.
CONCLUSIONS
In conclusion a standardized extract of Cupressus arizonica pollen in cases in which the major offender is likely to be Cupressus sempervirens pollen provided a sound provided a sound and easy approach using the SLIT technique to treat patients presenting a respiratory allergy to Cupressaceae.
Obviously, the purification of the different pollens of the Cupressaceae family (Cupressus sempervirens, Cupressus arizonica, Juniperus communis, Juniperus oxycedrus, Thuja orientalis, ecc.) will provide extracts which will give better results; the preparation of "ideal" standardized extracts using for each patient a mixutre "a la carte" of the "major" and "minor" allergens involved in a peculiar case will be possible, if not yet attainable, after identification, sequencing, cloning and testing for biological potency of all the allergens.