INTRODUCTION
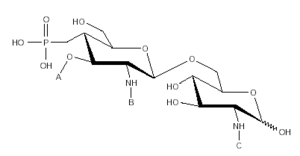
Inhaled allergens play a major part in respiratory diseases; moreover, epidemiological surveys indicate an increasing prevalence of allergic asthma and allergic rhinitis. Correspondingly, there is an ever-increasing need for effective treatment. Allergy Vaccination (AV), formerly known as Specific Immunotherapy (sIT), has been employed as a treatment strategy for these diseases for most of the 20th century1. AV is currently recognised as an efficacious procedure and able to bring about a more or less permanent remission after several years treatment (2). A number of measures that have been introduced to improve vaccine safety now include: optimal dosing, allergen modification (to reduce allergenicity whilst maintaining immunogenicity) 3, adjuvant adsorbtion (to control release) 4 and, very importantly, better standardisation and control of the products. Many allergy vaccines employ alum adjuvants which encourage a Th2 response in experimental animals 5; alum has also been shown to encourage strong IgE antibody responses in clinical studies 6. However, an optimal allergy vaccine adjuvant would promote a Th1 response which should result in a more significant reduction of clinical symptoms. This type of beneficial immunomodulation has been demonstrated using a new adjuvant, 3-deacylated monophosphoryl lipid A (MPL®adjuvant, Corixa), derived from Salmonella minnesota R595 7. The chemical structure of this adjuvant (which contains three major congeners) is shown in figure 1. MPL® adjuvant was found to suppress a specific IgE response following antigen booster injections in rats 8. Clinical studies have endorsed the use of MPL® as an efficacious and well-tolerated adjuvant in an antimicrobial immunotherapy vaccine 9. MPL® adjuvant and the depot material L-tyrosine have been shown to be synergistic for Th1 cell stimulation 10.
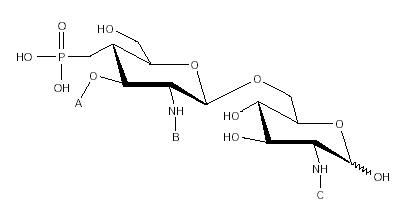
Figure 1.--Chemical structure of monophosphoryl lipid A.
The expectation of a more efficacious allergy vaccine from the inclusion of MPL® adjuvant has prompted its clinical application as an additional component of modern allergy vaccines such as Pollinex® Quattro (Allergy Therapeutics Ltd). These formulations incorporating MPL® for the AV of grass-pollen allergic patients have recently been investigated with demonstrated success in clinical trials 11. Treatment with Pollinex® Quattro comprises an initial course administered pre-seasonally using three subcutaneous injections of increasing strength followed by a fourth injection of the third strength.
This vaccine is made in an establishment subject to inspection by the Medicines Control Agency (MCA) and under Good Manufacturing Practice (GMP). The requirements to ensure high quality Therapeutic Allergy Vaccines are encompassed in the EU Notes For Guidance 12 and in the Ph.Eur. monograph Producta Allergenica13 and are followed in the manufacture and control of this product. Specifications, target values and stability are amongst the most important requirements in standardisation of the potency of the products and intermediates to ensure batch-to-batch consistency. The analytical methods and strategy applied at various stages of the manufacturing process to monitor batch-to-batch reproducibility and provide final product release are described here.
MATERIALS AND METHODS
Vaccine raw materials
All components are purchased from audited suppliers and supplied with certificates of analysis which must meet predetermined specifications. The tree pollens used in the allergy vaccines are from: Birch (Betula spp), Alder (Alnus spp), Hazel (Corylus spp). The pollen specifications include: water content, identity and lower limit levels of other pollens, other plant parts and mould spores. Following aqueous extraction of an equal mixture by weight of the pollens, the extract is vigorously monitored via biochemical and immunological assays. Examples of the examination of the pollens are described here. The addition of MPL® adjuvant requires particular scrutiny and we describe the assay of concentration and the congener composition as determined by HPLC. MPL® adjuvant is monitored using this assay methodology at all stages of manufacture from raw material status through to incorporation in the final product vaccine.
Methodology
Isoelectric focussing (IEF)
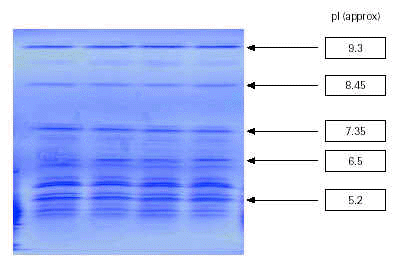
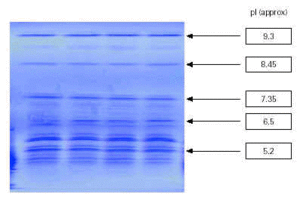
Aqueous extracts of the tree pollens were analysed qualitatively to provide high resolution of constituent proteins. Samples were run on an LKB Multiphor 2,117 using an Amersham Pharmacia Ampholine polyacrylamide gel (PAG) plate, pH 3.5-9.5 (T = 5 %, C = 3 %, ampholine concentration = 2.2 % w/v). IEF is a qualitative method of analysis from which a product specific fingerprint profile of the resolved constituent allergen proteins based on pI (isoelectric point) values is obtained. This is compared with an in-house reference preparation to ensure identity, batch-to-batch reproducibility and standardisation.
Crossed immunoelectrophoresis (CIE)
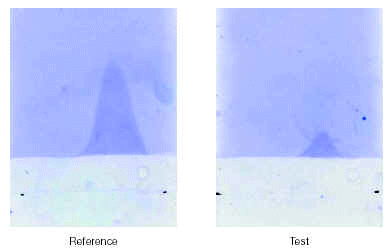
This technique allows measurement of major allergen content. Samples of pollen extract are electrophoresed (first dimension) on an LKB Multiphor 2117 using an agarose/PEG 8,000 gel in Tris buffer. Antiserum containing specific antibody to the allergen to be measured is added to the second dimension gel prior to solidification. For tree pollens, the content of the principle birch pollen allergen, Bet v 1, is found using polyclonal rabbit antiserum. The electrophoresed antigen complexed with antibody is visualised with Coomassie Blue stain and the area measured. An in-house freeze-dried birch pollen extract reference standard is used for comparison which is ascribed 1,000 Quality Assurance Units (QAU/ml). Thus the strength of the starting extract can be quoted in QAU/ml. Potency of the aqueous extract can be converted from QAU into mass units of major allergen, if required.
SDS-PAGE/Immunoblotting
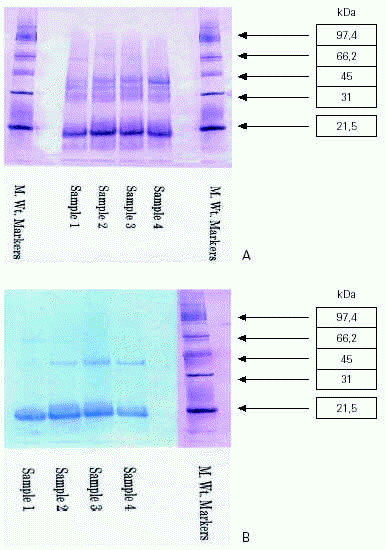
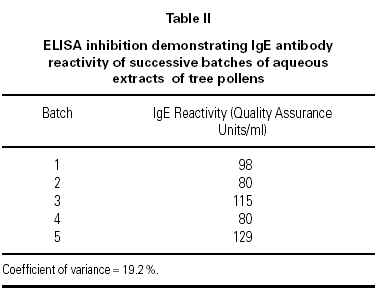
Batch-to-batch profile consistency is confirmed by analysis of each extract using SDS-PAGE. Protein molecular weight markers are compared, utilising a range up to 97.4 Kd (Bio-Rad Low Range SDS-PAGE standards). Visualisation using a gold stain enables standardisation of the constituent proteins. The allergen profile is identified with SDS-PAGE immunoblotting (Western blot) utilising a pool of sera from patients allergic to tree pollen as an IgE probe. The blots are developed with biotin-labelled goat anti-human IgE followed by addition of streptavidin-peroxidase and visualised with the chromogenic substrate 3,3',5,5'-tetramethylbenzidine.
Specific IgE assay
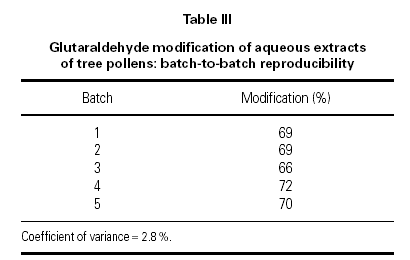
An ELISA inhibition assay based on a US Food and Drug Administration (FDA) assay 14 ensures that the allergenic activity (quantified as reactivity with specific IgE from tree-pollen allergic subjects) is reproducible from batch to batch. Comparisons are made with the in-house reference preparation and quoted in QAU/ml. Parallel line bioassay statistics are used to compute results.
The modified extract
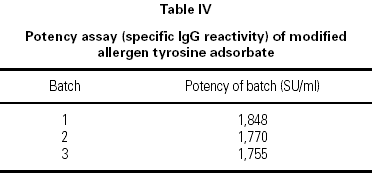
The aqueous pollen extract is modified by the addition of glutaraldehyde which cross-links proteins via the protein primary amine groups. Excess glutaraldehyde is completely removed by a dialysis system. The effect of modification is monitored using a specific fluorometric method 15 which is used to determine the concentration of primary amine before and after treatment. Hence the degree of modification can be calculated as a percentage. This modification results in a loss of IgE reactivity and thus provides increased clinical safety 16.
Final product formulation
The tyrosine-adsorbed, glutaraldehyde-modified bulk extract is prepared by co-precipitation of the modified allergen extract with L-tyrosine 4, 17. Various bulk dilutions are then prepared by diluting with blank L-tyrosine. MPL® adjuvant is then added to each dilution strength to obtain the required concentration of 50 mg/ml. The four-injection set of Pollinex® Quattro immunotherapy vaccines consists of three strengths which differ only in the concentration of modified allergen present. The strengths are: 300, 800 and 2,000 standardised units (SU)17 per ml. The top dose is repeated for the final injection. All three strengths are composed of 2 % w/v tyrosine adsorbates containing glutaraldehyde-modified allergen with 50 mg/ml MPL® adjuvant in aqueous excipients consisting of 0.5 % phenol, 0.9 % sodium chloride and 0.4 % phosphate buffer.
The inactive ingredients and sterility of the vaccines are measured using standard techniques. Tyrosine adsorbate analysis was as described previously 4, 16.
Potency assay (antigenic recognition by specific IgG)
The potency assay of the final product relies on the ability of the pollen extract to react with specific IgG antibody which is essentially retained after modification 16. An IgE-based assay would not be appropriate because the modification results in a very much-reduced reactivity with specific IgE antibody. Dilutions of solublised vaccine product are adsorbed to the wells of a microtitre plate. Specific polyclonal rabbit antisera to tree pollen 17 at an appropriate dilution is reacted with the product protein, washed and followed by europium-labelled protein A which reacts with the bound IgG antibody 18. The antibody binding is quantified by time-resolved fluorescence and the product potency calculated against a reference preparation. Once more a comparison with a reference extract is made in order to provide potency values in an allergy vaccine unit, the Standardised Unit (SU).
Regulatory authorities require that consistent adsorption must be proven in the case of allergy vaccines utilising depot adjuvants such as L-tyrosine. This is achieved by assaying the supernatant component of the product, and deemed acceptable if the levels are below the standard that has been validated to equal the dose required to induce a skin test reaction.
MPL® adjuvant assay: chemical structure
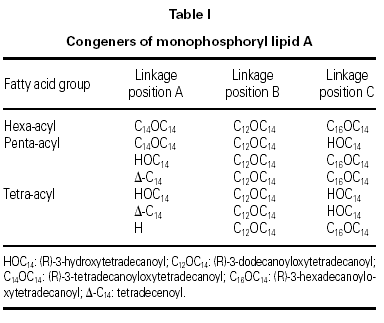
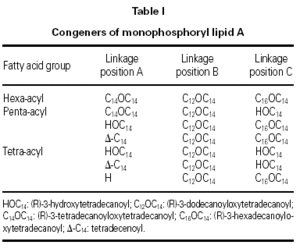
MPL® adjuvant (fig. 1 and table I) is composed of a mixture of species all having the same disaccharide backbone, but differing in terms of fatty acid substitutions at positions A, B and C. Some substitutions contain a second fatty acid, terminating at the 3-position of the first. MPL® containing a total of 4 fatty acid groups is known as the tetra-acyl congener species, 5, penta-acyl and 6, hexa-acyl.
MPL® adjuvant assay: aqueous formulation and intermediate preparations
The concentration and congener composition of the raw material MPL® adjuvant aqueous formulation (MPL-AF) as supplied by the manufacturers and the 0.1mg/ml Intermediate bulk dilutions are determined by reverse phase HPLC assay on a Waters Symmetry C18 column, particle size 5 μm, dimensions 4.6 x 150 mm 19. A fluorescence detection system employed an excitation wavelength of 345nm and emission at 515 nm. A gradient elution protocol used mobile phases of: [A] 5 mM tetrabutylammonium phosphate (TBAP), in acetonitrile-water (95:5, v/v), [B] 5mM TBAP in isopropanol-water (95:5, v/v). Elution of the congeners is accomplished by applying a linear gradient of 10 % to 80 % [B] over 45 minutes. Since MPL® adjuvant is not a good chromophore, spectrophotometric detection sensitivity is improved by derivatisation with dansyl hydrazine (a fluorescent tag) prior to analysis.
The total MPL® content and congener composition of the test sample (prior to the addition to the vaccine formulation) is established by comparison of various peak areas on the chromatogram with those from a 20 mg aliquot of derivatised MPL® adjuvant standard. The values obtained are compared with those given on the certificate of analysis from the manufacturer (raw material) or the in-house specification (0.1 mg/ml intermediate).
MPL® adjuvant assay: the final MATA vaccine product (Pollinex® Quattro)
The same method employed for the MPL-AF preparations was also used to standardise the MPL® adjuvant component in the final product, but with one additional step. The L-tyrosine adsorbate components interfere in the MPL® analysis and must therefore be separated from the MPL® adjuvant component before derivatisation. This is facilitated by first solublising the L-tyrosine adsorbate using 1M HCl and then solvent extracting the MPL® component using chloroform/methanol 2:1 v/v. The MPL® component can then be derivatised and analysed using the same HPLC method employed for the MPL-AF raw material and intermediate.
RESULTS
Isoelectric focussing
Figure 2 shows the high degree of batch-to-batch consistency obtained from aqueous extracts of different batches of birch pollen.
Figure 2.--Identification test: isoelectric focussing pattern of four batches of birch pollen extracts.
Crossed immunoelectrophoresis (CIE)
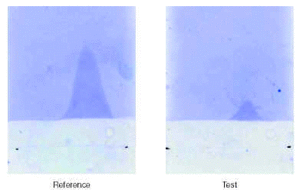
A test sample is compared to a reference standard as shown in figure 3. Quantification is achieved by estimation of the stained area of the sample in relation to the area given by the reference to obtain a figure expressed in QAU per ml.
Figure 3.--CIE quantification of the major allergen, Bet v 1, in aqueous birch pollen extract.
SDS-PAGE immunoblotting
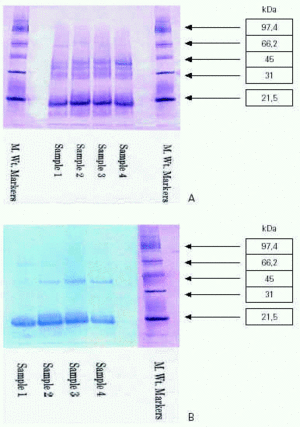
The similarity in the profiles of the four batches of tree pollens confirm batch-to-batch reproducibility with respect to protein composition (figure 4a) and human IgE reactivity (figure 4b).
Figure 4.--SDS-PAGE analysis of aqueous tree pollen extracts from four successive batches. (A) Protein profiles using gold staining. (B) Allergen profiles using Immunoblotting Against Human IgE.
Specific IgE assay (ELISA)
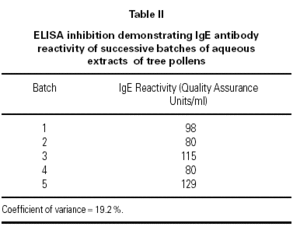
Five batches of pollen extract prior to modification were tested for specific IgE reactivity, as shown in table II. Results were well within the specification range that is stipulated in the EU Notes for Guidance 9.
The modified extract
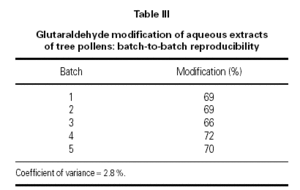
Five batches of pollen extracts were assayed for primary amine content before and after modification with glutaraldehyde and the proportion of primary amine groups modified is shown in table III. These are well within the range that is consistent with the required safety bonus conferred by glutaraldehyde modification.
Potency assay (antigenic recognition by specific IgG antibody)
Table IV shows the analysis of three separate batches of the final product containing MPL® adjuvant. The results, quantified in SU/ml, indicate good batch-to-batch reproducibility, which falls well within the required specification range.
MPL® adjuvant HPLC assay
The MPL® adjuvant content and congener composition is determined on a concentrated aqueous solution (raw material), a diluted intermediate and on the Pollinex Quattrofinal product.
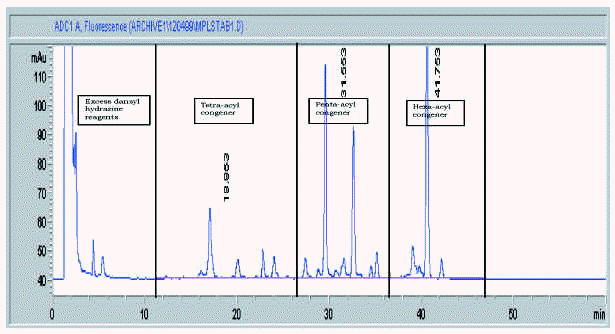
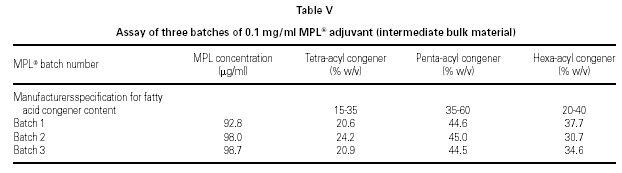
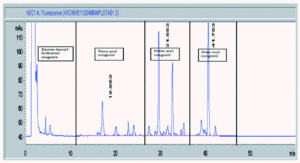
Figure 5 shows a typical chromatogram of a 20 mg sample of an MPL® adjuvant raw material batch prior to dilution, indicating the position of the three congener windows. The total MPL® adjuvant content and congener composition are established by comparison of the peak areas of a 20 mg aliquot of a dansylated derivative of an adjuvant standard with those of the sample. The manufacturers specification is shown in table V and must be met at all stages (the raw material, the bulk intermediate stage and in the final product formulation).
Figure 5.--HPLC profile of aqueous formulation of MPL® adjuvant, raw material.
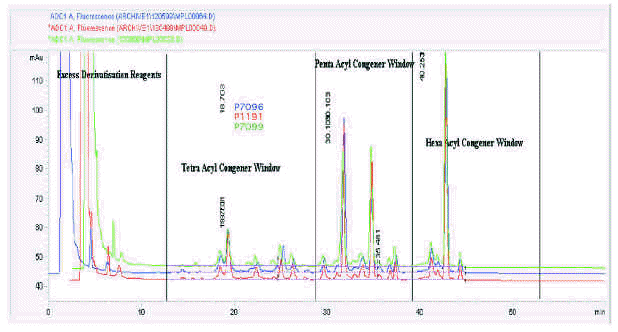
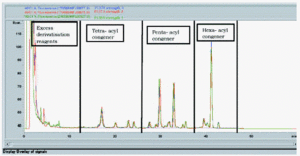
Batch-to-batch consistency is shown on figure 6 for three batches of 0.1mg/ml MPL® AF bulk intermediate prepared from three different raw material batches at different times and analysed on different HPLC runs. Given the amount of fine structure observed in a typical MPL® chromatogram, it is notable that the profiles are qualitatively almost identical.
Figure 6.--MPL® assay: a trace overlay comparison of three different batches of 0.1mg/ml MPL® raw material.
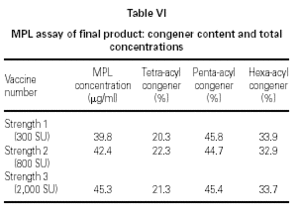
Table VI shows the MPL® adjuvant concentration and congener composition obtained from the chromatogram shown in figure 6 for the three different 20 mg batches of adjuvant. These are typical examples of batch-to-batch consistency.
MPL® adjuvant assay of Pollinex® Quattro
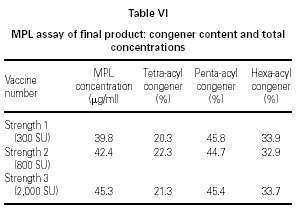
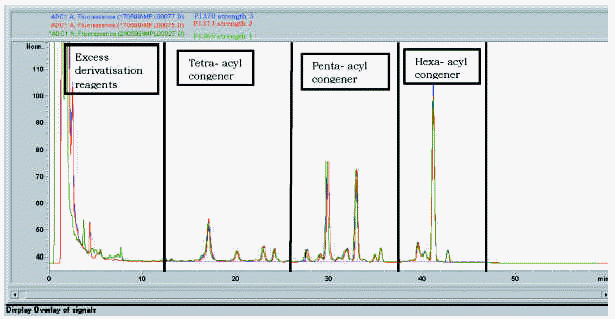
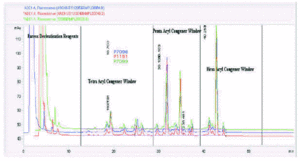
A treatment course incorporates three strengths of modified allergen extract. Figure 7 shows the elution profile and table VI the MPL® content and congener composition for a set of final product vaccines. In figure 7, the traces have been overlaid to show that the fine structure is identical despite the differing allergen content. In table VI, the MPL total values are consistently slightly under the theoretical 50 mg/ml concentration; the discrepancy is attributed to less than 100 % solvent extraction efficiency in the HPLC sample work-up. These results indicate that the MPL® congener composition is immediately stable within the vaccines and that the allergen content does not affect the MPL® adjuvant component measurement.
Figure 7.--MPL® assay: a trace overlay comparison of three strengths of Pollinex Quattro vaccine.
DISCUSSION
Novel allergy vaccines such as Pollinex® Quattro have been developed to reduce the number of injections required to treat patients effectively which increases the attraction of this form of therapy for type 1 respiratory hypersensitivity. The efficacy of these vaccines is assisted by the inclusion of MPL®, a powerful Th1-inducing adjuvant. The short course design requires a rapid dose escalation and hence an allergoid was used to counteract potential allergic side-effects of this form of therapy.
Such products must be standardised according to regulatory requirements 12, 13 and an overall strategy has been suitably established; excipients are controlled by standard Quality Control assays. The active ingredient (in this paper, allergens from tree pollen) is kept within specification at all stages of the manufacturing process by validated allergen assays. The modified allergen in the final product was assayed as an antigen by specific IgG; an IgE assay would be innapropriate owing to the effective epitope loss by chemical modification of the allergens. The specific IgG assay employed europium as a label, which is a relatively novel procedure and appears particularly user-friendly. Radioimmunoassays are complicated by various safety and regulatory issues and diminution of radioactive isotope half-life, whereas the use of enzyme-labelling (ELISA) can sometimes result in poor sensitivity and unwanted enzyme inhibition effects.
The major challenge for the standardisation of Pollinex® Quattro was the development of an assay method to monitor the MPL® adjuvant component both before and after incorporation into the tyrosine adsorbate. MPL® adjuvant is not a single chemical entity, but a mixture of analogues, with the differences reflected in the number and length of fatty acid chains. This complex problem was resolved by exploiting the high resolution and assay accuracy now made possible by HPLC technology. It was found that MPL® adjuvant content and its congener composition can be consistently and accurately measured by these methods. These assays and the results are not affected by the vaccine components components or vice versa.
In conclusion, clinicians using this new vaccine product can be assured that different batches will be consistent with respect to both the active modified allergen and the novel adjuvant. Analogous products in this new range for allergy to other allergens (eg grass pollens and house dust mite) are controlled and standardised by similar methods.
ACKNOWLEDGEMENTS
The authors wish to thank Corixa Corporation, Seattle, Washington, USA and especially Dr Terry J. Ulrich, Dr. William D. Pfeffer and Dr Tom Crane for all their help and advice with the MPL® adjuvant HPLC assay. We also thank Domingo Vidal Echauri and Isabel Riera Galiana (Allergy Therapeutics Ibérica, Barcelona, Spain) for their help with the manuscript.
MPL® is a registered trademark of Corixa Corporation, Seattle, Washington, USA. All other trademarks referred to in this document are the property of their respective owners.