INTRODUCTION
Nasal provocation tests (NPT) have been extensively used in clinical practice to evaluate chronic rhinopathies, mainly allergic rhinitis. Its effectiveness in the identification of a probable etiological allergen is similar to bronchoprovocation tests. However, it is safer and easier to perform. Nowadays, the NPT have been used in the evaluation of nasal response to specific provocative agents (polens, mites or molds) or to non-specific agents (histamine or methacholine), reproducing clinical picture. It has been objectively evaluated by nasal resistance measurement, quantitative and/or qualitative cellular influx analysis, and mediator's levels. Also, it effects maybe subjectively determined by the evaluation of challenged symptoms (itchy nose, sneeze, coriza and obstruction) or a specific score (1).
NPT also allows: to study the action of specific drugs (anti-histamines, anti-cholinergics, intranasal corticosteroids, etc); to induced infections and to study their consequences under controlled conditions (virus inoculation); and, finally, to confirm the etiological agent when skin prick test were of difficult interpretation (2, 3).
The objectives of this study were to evaluate the reproducibility of histamine (H) NPT and to standardize specific NPT with Dermatophagoides pteronyssinus (Dp) and Blomia tropicalis (Bt), in subjects allergic to them. It was also investigated naso-pulmonary reflex, the correlation between cutaneous and nasal reactivities to both allergens, and the effectiveness of a combined score (clinical and rhinomanometric) as an auxiliary method in the interpretation of NPT.
PATIENTS AND METHODS
The studied group comprised 10 children (5 boys), aged from six to 15 years (mean age = 10 years), followed at the Division of Allergy, Clinical Immunology and Rheumatology of the Department of Pediatrics, Universidade Federal de São Paulo-Escola Paulista de Medicina. This study was approved by the Ethical Medical Committee and all patients had a signed informed consent by their parents or guardians.
All children had perennial allergic rhinitis (anamnese and physical examination) and a positive skin prick test (SPT, mean wheal diameter ≥ 3 mm) to inhaled allergens: Dp (112,900 BU/ml; IPI-ASAC, Brazil) and Bt (1 mg/ml; yielded by Dr. Enrique Fernadez-Caldas, Spain). The SPT were performed with the same allergen dilutions employed in NPT. A negative (saline plus 0,5 % phenol) and positive (histamine 10 mg/ml) controls were used in the SPT.
After admission, the patients were submitted to NPT, always in the morning and after acclimatization in a room with controlled temperature (24-6 °C). NPT were performed two weeks apart (first Dp and second Bt) and in two different days, always preceeded by provocation with histamine (H).
NPT was monitored by anterior active rhinomanometry (Berger Rhinomanometer S/A, SP, Brazil). Total nasal resistance (TNR) was obtained calculating the resistance of each nostril separately (P/V): TNR = RightNR xLeftNR / RightNR + LeftNR (4, 5).
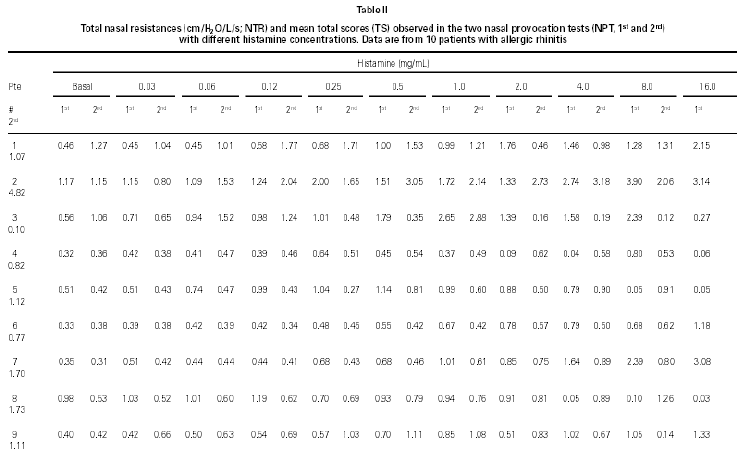
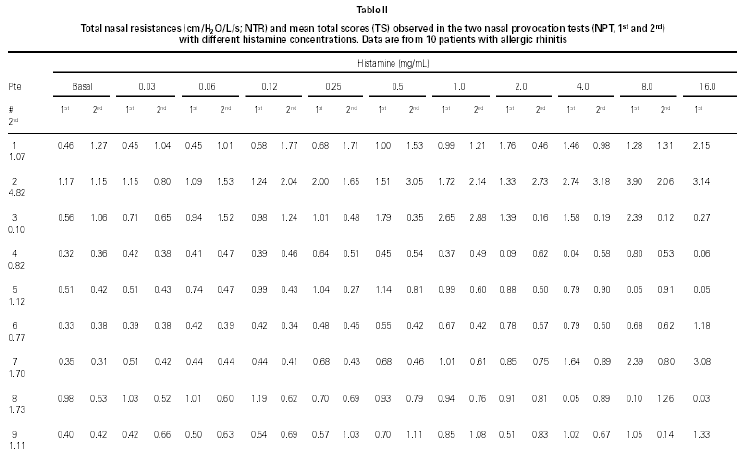
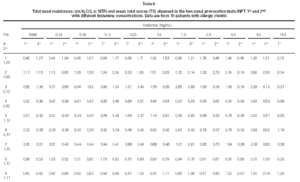
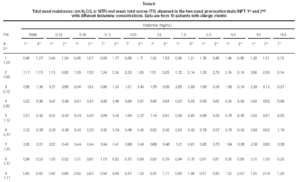
Non-specific NPT with H was carried out as previously standardized by our group (6). Briefly, drugs that might interfere with NPT were excluded. After basal TNR was obtained, each nostril was aspersed with 0.1 ml of saline and five minutes latter a new TNR was determined, and this was considered NPT's reference value. After this determination, 0.1 ml of increasing H concentrations (0.03; 0.06; 0.12; 0.25; 0.5; 1.0; 2.0; 4.0; 8.0; 16.0 mg/mL) were instilled in both nostril each five minutes and a new TNR measurement were obtained. Pulmonary function tests were performed to obtain forced expiratory volume in the first second (FEV1) and forced expiratory flow at 25 to 75 % of the forced vital capacity (FEF25-75 %). All patients were challenged with all dilutions even when the positive provocative concentration (increase 100 % in TNR reference) was reached. A 20 % decrease in basal FEV1 and FEF25-75 % values were considered as a positive pulmonary response (7). At the same time, we determined the total score (table I) which was considered positive when equal or superior to 5 (8).
Specific Dp and Bt NPT were carried out 24 hours after NPT with H. The initial steps were similar to H NPT. The instilled concentrations were obtained from the raw extracts of Dp (112,900 BU/mL-1/1 weight/volume): 1/100,000; 1/10,000; 1/1,000; 1/100; 1/10; 1/5 and 1/2.5 and of Bt (1 mg/mL-1/1 weight/volume): 1/125,000; 1/25,000; 1/5,000; 1/1,000; 1/200 and 1/10. Increasing concentations were instilled in each nostril at five minutes intervals.
Serum levels of specific IgE to Dp and Bt (Pharmacia CAP System RAST- FEIA, Pharmacia Diagnostics) were determined in all patients.
Statistical analysis were performed by non-parametric tests: Friedman's variance analysis by ranks and Wilcoxon's test. The level for rejection of the null hypothesis was fixed at 5 % (alpha < 0.05), and significant values were indicated by an asterisk.
RESULTS
NPT with histamine was positive in both provocations in 8/10 children (table II). Other two patients had once a positive NPT with H. One of them had a positive Dp NPT after the H NPT (patient # 1) and the other had a positive NPT with Dp without a positive H NPT and a positive NPT with Bt after a positive NPT with H (patient # 8).
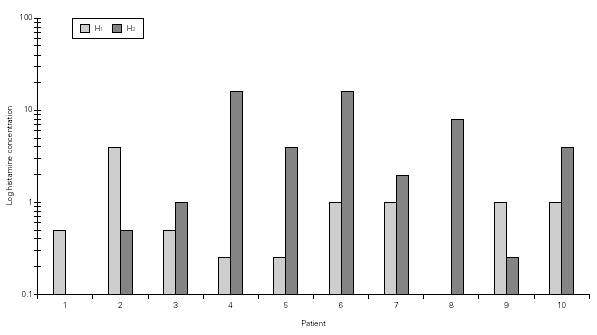
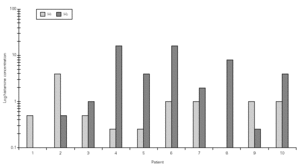
Considering the whole group, in both H NPTs we observed a significant increase in TNR starting from 0.5 mg/mL H concentration. Same was observed regarding TS. No differences between the two H NPT were observed according to TNR and TS (table II). Considering the doubling dose, we observed that only two patients (# 3 and # 7) had the same H concentration as inducing a positive NPT (fig. 1). There were no significant differences between mean TS and mean TNR observed in both NPT with H (table II).
Figure 1.--Histamine concentration that induces a positive nasal provocation test in two tests a week apart (H1 and H2).
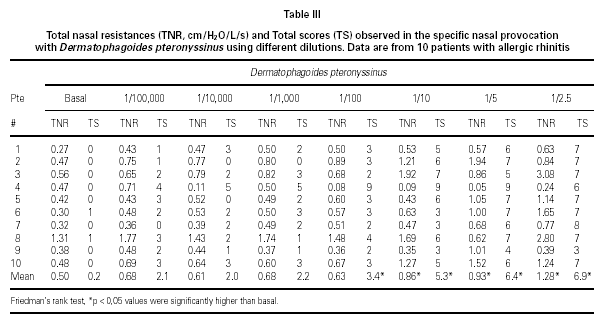
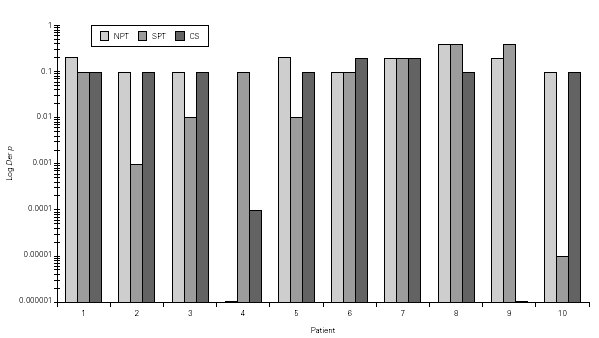
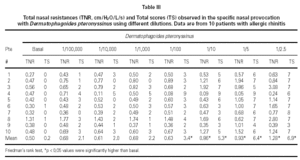
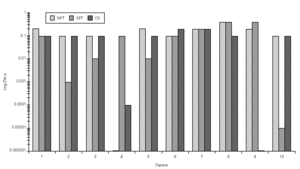
Dp NPT showed a significant increase of the mean TNR in comparison to basal value up to Dp 1/10 concentration (table III). Considering TS a significant increase was observed up to Dp 1/100 concentrion (table III). However, when we used the NPT positivity criteria (100 % basal TNR increase), Dp NPT was positive in 9/10 patients (4/9 in concentration 1/10; 4/9 in 1/5 and 1/9 in 1 / 2.5; table III; fig. 2).
Figure 2.--Concentration of Dermatophagoides pteronyssinus (Der p, log weight/volume) that have induced a positive nasal provocation test (NPT), skin prick test (SPT) and Combined score (CS).
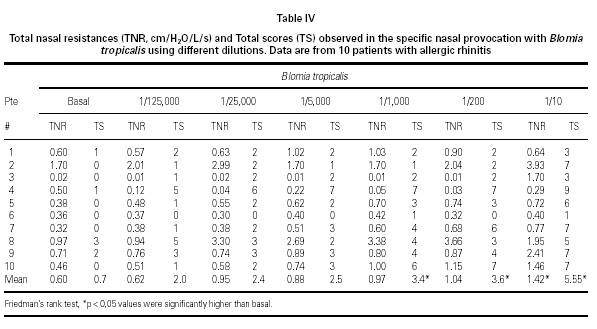
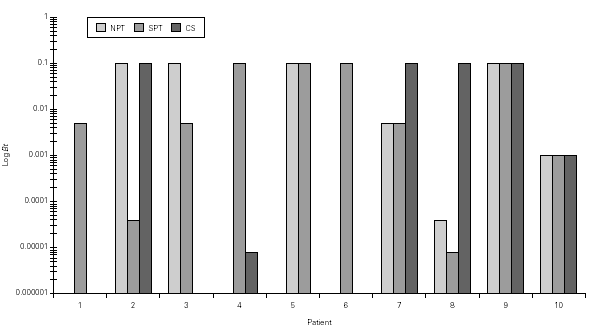
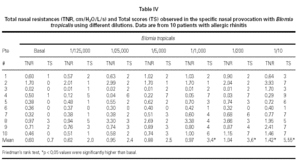
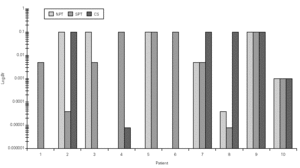
Mean TNR increased significantly in comparison to basal value up to Bt 1/10 concentration during Bt NPT (table IV). Mean TS increased significantly, considering basal values, up to Bt 1/1,000 concentration (table IV). However, when was considered the NPT positivity criteria Bt specific NPT was positive in 6/10 children (1/6 in concentration 1/25,000; 1/6 in 1/1,000; 1/6 in 1/200 and 3/6 in 1/10; table IV; fig. 3). Only one patient presented negative tests for both mites.
Figure 3.--Concentration of Blomia tropicalis (Bt, log weight/volume) that have induced a positive nasal provocation test (NPT), skin prick test (SPT) and Combined score (CS).
There was no concordance between Dp and Bt concentrations that induced positive NPT and positive SPT and positive TS (figs. 2 and 3).
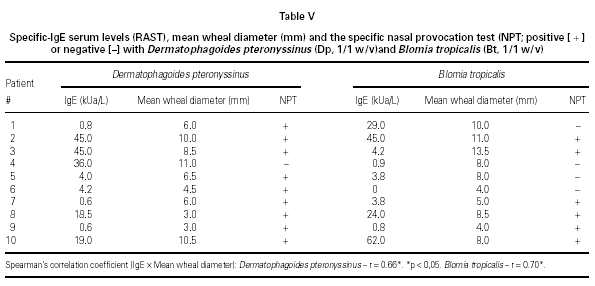
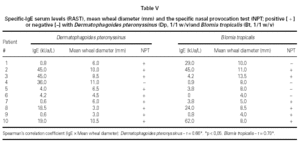
There was a significant correlation between serum specific IgE levels and SPT for both mites, independently of their concentrations (table V). The concordance index (both positive or negative tests) varied from 60 % to 90 % for both mites. During all the NPT it was not observed a significant fall in the pulmonary function measurements.
DISCUSSION
NPT with histamine is a task tool with high sensitivity (93 %) and specificity (83 %) for the diagnose of nasal hyperresponsiveness (2). Although there is no consensus about its method regarding H concentrations, volume instilled, interval between instillations, evaluation criteria etc, in this study we have used a previously standardized method by our Division (6) added of two new H concentrations (8.0 and 16.0 mg/mL). In general, the H concentration that is pointed out as the concentration able to distinguish individuals with and without nasal hyperresponsiveness are variable from 0.5 mg/mL (9) to 4 mg/mL (5, 10, 11). Thereby only in 3/18 positive H NPT, the provocative concentration was higher than 4 mg/mL.
NPT with H is a standardized method and has been broadly used in clinical investigation. However, its reproducibility has not been evaluated. Considering this observation, we have submitted such patients to H NPT in two different occasions. H NPT were positive twice in 80 % of patients. Patients # 1 and # 8 had once a positive H NPT. Analysing each nostril resistance individualy (data not shown), in both patients, we observed, after H nasal instillation, a strong increase in nasal resistance in one nostril reaching very high values (100 cm H2O/L/s), and a contralateral inverse compensatory action. Because of that, perhaps TNR has not surpassed the 100 % of basal TNR in any H concentration. Accomplish H NPT during the top nasal cycle in this nostril may be other explanation for this finding (12).
Considering 4 mg/mL H as cut-off concentration, 15/18 of the positive H NPT occurred bellow this cut-off concentration. There was a 67 % of agreement between the two H NPT. Considering the criteria of doubling dose, as in the evaluation of bronchial hyperresponsiveness, only 2/8 patients presented similar H provocative concentration in both tests (fig. 1). Mean TNR were similar in both tests.
Our results are similar to the obtained by Giannico et al (6) that observed a positive NPT in 19/23 atopic patients (82.6 %), and it started from the 0.5 mg/mL H concentration. Symptoms have been used to monitoring NPT, as an alternative way to sophisticated and expensive equipaments (6, 13). In this study TS (clinical and rhinomanometric) and TNR showed a significant increase up to 0.5 mg/ml of H in both NPT (table II and fig. 1).
NPT with Dp were designed based on previous study including two other Dp concentrations (1/5 or 22.58 UB and 1/2.5 or 45.16 UB) (1). Considering the mean NTR values, there was a significant increase of basal mean NTR up to the 1/10 Dp concentration during Dp NPT. However, positive Dp NPT was observed only in 1/2.5 Dp concentration (table III). Analyzing each patient apart, Dp NPT was positive in 40 % patients at 1/10 concentration, in 40 % at 1/5 and in 10 % at 1/2.5, reaching 90 % of positive Dp NPT. Similar to our data, Fernandes et al had observed 90.6 % of positive Dp NPT and their provocative concentration were bellow 1/100 for all patients studied (10). Olivé-Perez reported 95 % of positive Dp NPT in patients sensitive to Dp (11).
Analysing Dp concentration capable to induce a positive NPT, based on TNR and TS, only 4/9 of them had positivity of these parameters in the same Dp concentrations. In 3/9 the TS positivity occurred in lower Dp provocative concentration lower than those of NTR (fig. 2).
Bt NPT was positive in 60 % of patients, and a significant increase in the mean TNR and TS positivity was observed in Bt 1/10 concentration. However, mean TS have increased significantly up to Bt 1/1,000 concentration. Analysing each patient apart, there was concordance between the Bt concentration that induced positive NPT evaluated by TNR and by TS only in two patients (fig. 3).
TS show no advantage on TNR in monitoring non-specific and specific NPT.
Stanaland et al. (14) submitted Bt sensitive PAR patients to Bt NPT, using similar concentrations to those utilized in this study, except for 1/10. They observed 83 % of positive NPT. Arruda et al (15) evaluated patients with exclusively Dp or Bt sensitives and by a imuno-absorption study founded cross-reactivity between both mites in 36 %. We could speculate that a positive Bt skin test could be attributed to cross-reactivity with Dp, but the mucosal response would be more specific.
Regarding to SPT, the majority of studies have compared results observed in nasal mucosa with the SPT positivity or not to the same allergen, without considering the concentrations utilized. The indexes of concordance between both exams have been variable depending on the involved allergens. Daele and Melon (13) observed 52 % of concordance for house dust, Clarke (17) 81 % for Dp and Lebel et al. (18) 92 % for grasses. Our study showed a concordance of 90 % for Dp and 60 % for Bt, since all the patients are sensitive to both mites.
The stimulation of receptors in nasal mucosa, in nasopharynx, and in paranasal sinus, in animals and humans, have been followed by bronchoconstriction (19). Similar to others, we do not observed pulmonary function test changes in both NPT specific and non-specific (6, 10, 20).
In conclusion, the high level of concordance between the H provocative concentration obtained in both H NPT reinforces that it is a reproducible test. TS shows positivity simultaneously to the TNR and does not seem more advantageous than TNR in evaluating NPT. There was a weak positive correlation between mean wheal diameter and serum specific IgE levels to Dp and Bt (21). In spite of serum specific IgE (RAST) and SPT are equally effective in the etiological diagnosis of allergic rhinitis, specific NPT might be applied when a discrepancy between clinical history, symptoms, SPT and/or serum specific IgE occur.