Previous studies have shown that serum interleukin 33 serving as an “alarmin” is increased in children with asthma. The objective of this study was to assess the validity of serum IL33 test for early diagnosis of childhood asthma.
MethodsA literature search was performed in June 2016 using PubMed, Embase, the Cochrane Library and other Chinese Medical Databases to identify studies. The search terms used were “cytokine”, “interleukin-33“, “asthma” and “children”. The meta-analysis was performed using Review Manager 5.3 software. Random-effects model was used to estimate the standardized mean differences (SMDs) with 95% confidence intervals (CIs).
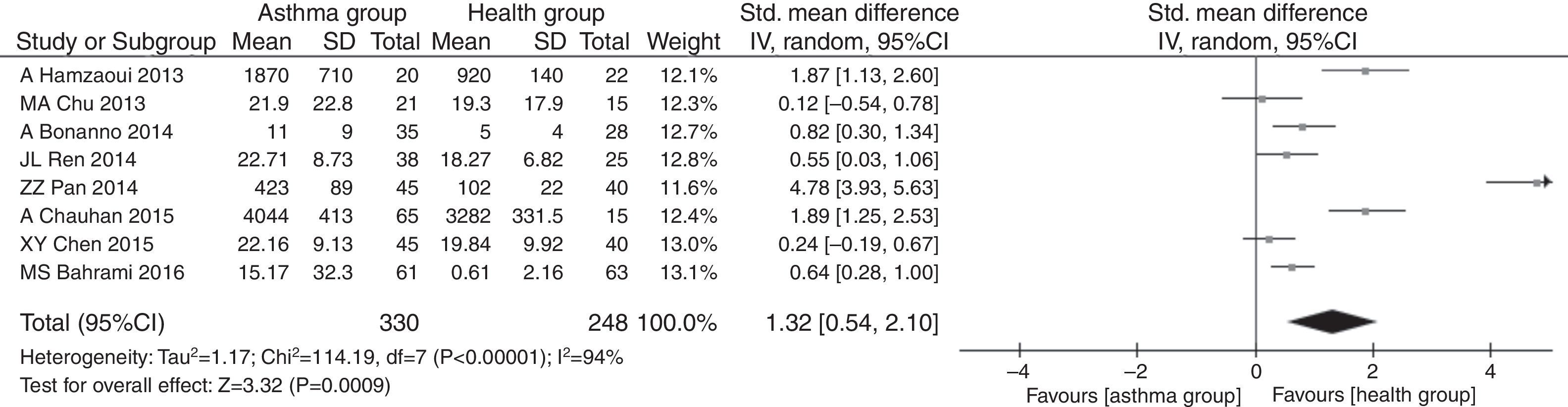
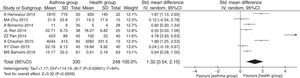
ResultsA total of eight studies were included into this meta-analysis, involving 330 asthmatic children and 248 healthy children. The meta-analysis results revealed that the serum IL33 level was higher in asthmatic children compared to that in healthy children (SMD=1.29, 95%CI=0.53–2.05, P=0.0009), with significant heterogeneity across studies (I2=94% and P<0.00001).
ConclusionsThe meta-analysis showed that serum IL33 is a helpful biomarker for early diagnosis of childhood asthma. However, owing to lack of enough data, the increased serum concentration of IL33 cannot be an indicator for the asthma severity.
Asthma is a chronic inflammatory disorder of the airways, characterized by periodic attacks of wheezing, shortness of breath, chest tightness, and coughing.1 The prevalence of asthma is increasing in most countries, especially among children.2 Childhood asthma is more common in boys than in girls and may persist throughout life. Because asthma symptoms are heterogeneous in children, persistent wheezing and respiratory symptoms in children may indicate other diagnostic considerations. Unnecessary investigations can delay the diagnosis of childhood asthma. In some cases, uncontrolled asthma without proper treatment can be fatal.3
IL33 is IL-1 family constitutively expressed by many cell types included bronchial and epithelial cells, endothelial cells, macrophages and dendritic cells.4,5 Recent studies emphasize IL-33 possibly serving as a danger signal (“alarmin”) to activate the immune system following cell aggression and damage.6,7 After genome-wide association studies, strong associations between single nucleotide polymorphisms (SNPs) flanking IL33 gene and asthma have been approved.8,9 However, the association between the level of serum IL33 and childhood asthma is unknown. Although some studies reported significant elevations in serum IL33 levels in childhood asthma compared with health controls,10–15 these were not confirmed in similar studies.16,17 Moreover, it is unknown whether the level of serum IL33 can be an indicator for childhood asthma symptom severity.
Thus, the purpose of this meta-analysis was to analyze the level of serum IL33 in childhood asthma, which may be a marker of the disease severity and potential clinical diagnosis targets.
MethodsLiterature search and selection of studiesA literature search was performed in June 2016 using PubMed, Embase, the Cochrane Library and other Chinese Medical Databases to identify studies. The search terms included “(IL33 OR Interleukin-33)” AND “(asthma)” AND “(children and infant)”. The searches were restricted to studies of only human subjects, and no language restrictions were applied. The reference lists and supplemental materials associated with the studies and review articles were examined manually to further identify any additional relevant publications.
To minimize this potential bias, we also investigate other sources of information. First, we hand searched the relevant conference proceedings for the previous 10 years in the following sources: Annual Pediatric Asthma Conference, Pediatric Allergy and Asthma Meeting, World Pediatric Congress, International Conference on Pediatric Allergy, Immunology and Pulmonary Disease, International Conference on Respiratory System Diseases, and Academic Conference on asthma in China. Second, we searched the Internet through search engines Google.com and Baidu.com, using the term ‘clinical trial & IL33’. Third, we contacted researchers, graduate students in the field and pharmaceutical companies for information on unpublished and ongoing trials.
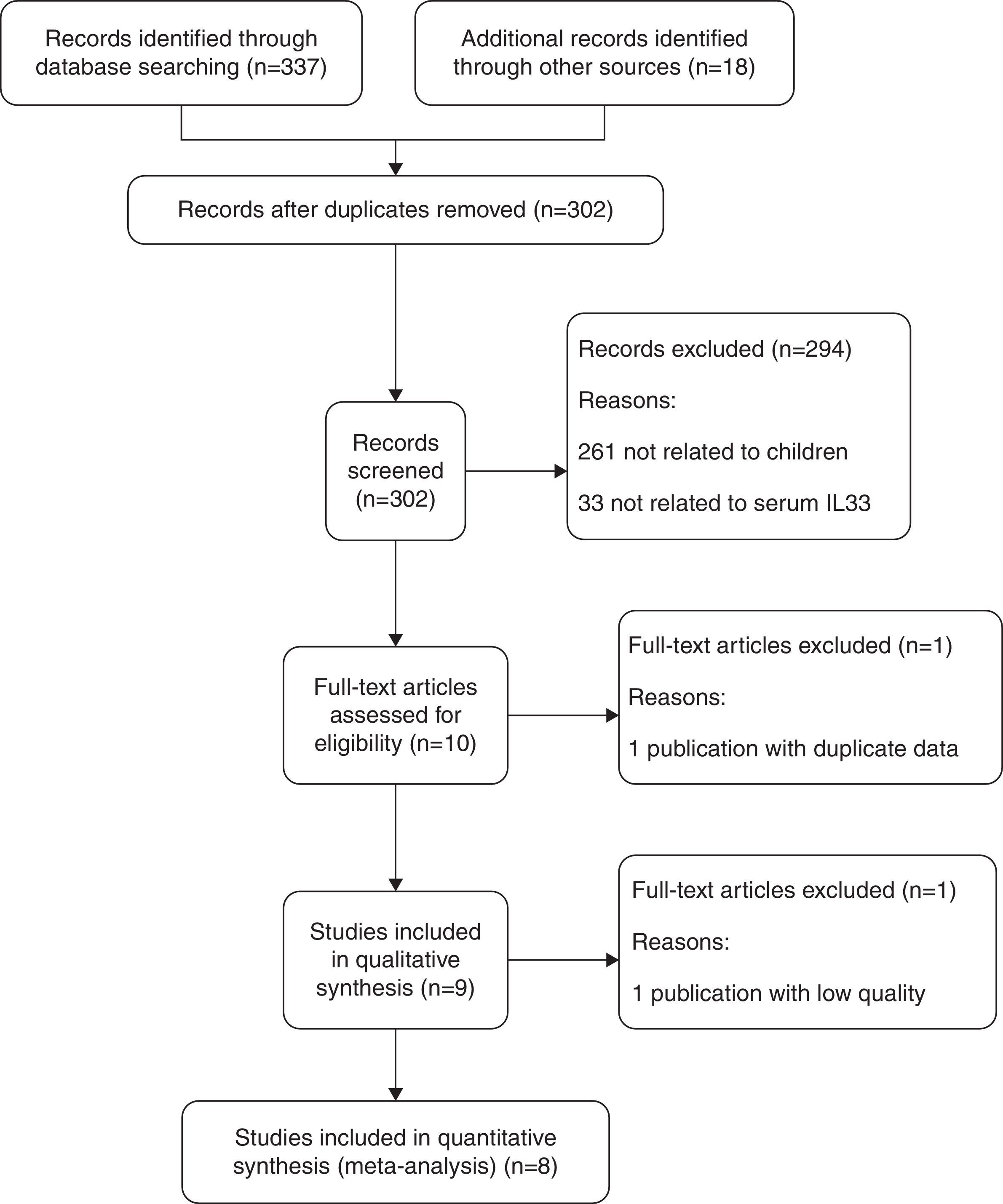
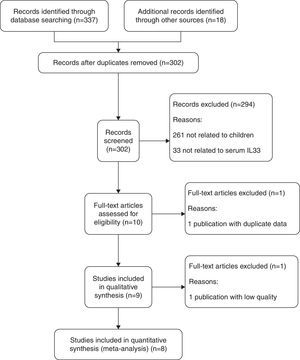
Those studies aiming to explore the association between serum IL33 expression and childhood asthma were included. The inclusion criteria of studies were: (1) children with asthma; (2) healthy children in control group; (3) the articles focused on the serum IL33 level; (4) full text, original research articles could be found. The following were cause for exclusion: (1) a lack of data regarding the serum IL33 level and childhood asthma; (2) not a primary case–control study; (3) insufficient data was extracted from the articles or the full text could not be found. The study inclusion and exclusion procedures are summarized in Fig. 1.
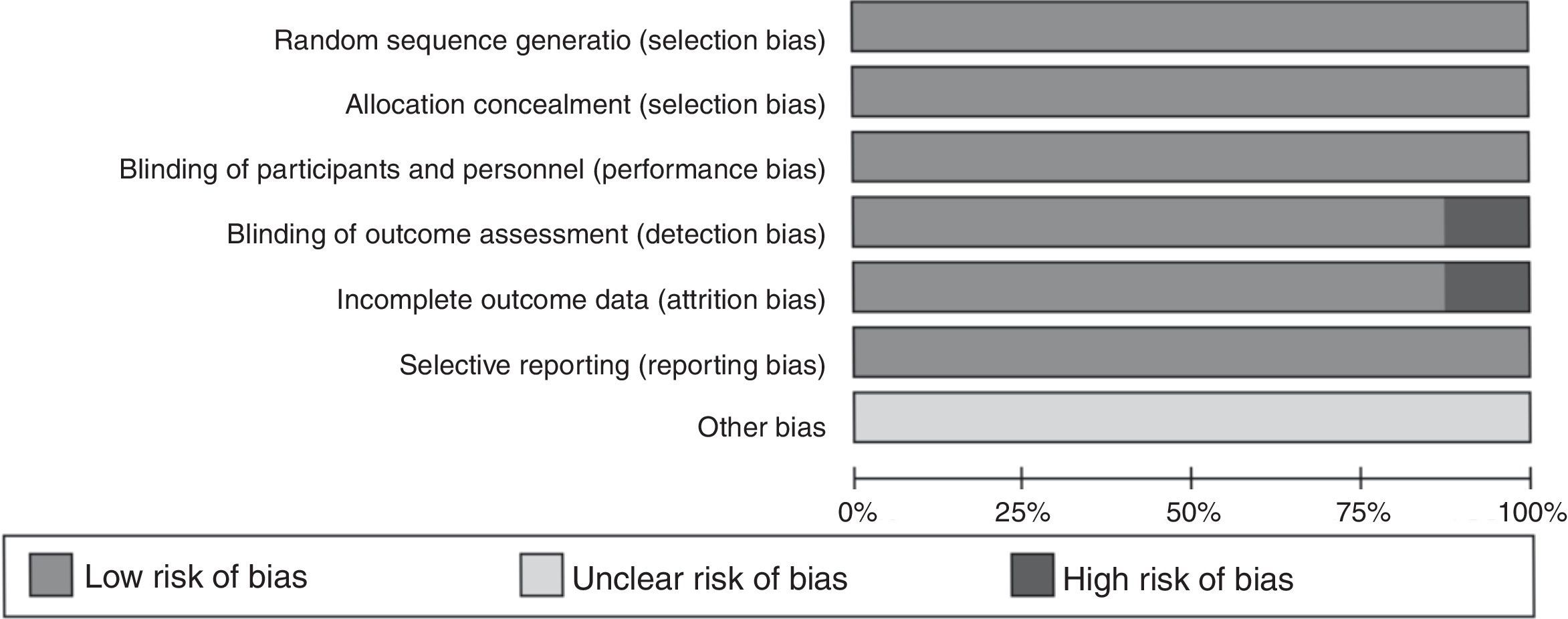
Data extraction and quality assessmentTwo investigators (Y Wang and L Wang) independently performed the data extraction. The general characteristics of the study were extracted using a standardized data extraction form: publication information (first author's name, publication year), characteristics of asthma and control (country, measure method, reagent source, study design, sample size, mean age), and outcomes (asthma severity addressing by the studies). Discrepancies were resolved by discussion with a third investigator (S Hua). The quality of studies was evaluated according to the quality assessment of diagnostic accuracy studies (QUADAS) tool including seven questions. Answers are “Low risk of bias”, “Unclear risk of bias”, and “High risk of bias”, respectively. If a study has more than four answers with a high risk of bias, it was considered a “low-quality” study. The “low-quality” study was excluded from the meta-analysis.
Statistical analysisThe meta-analysis was performed using Review Manager 5.3 software. Serum IL33 levels were extracted as the mean±standardized difference (SD) in each study. If the mean level differences were significant across studies, or different units were used, standardized mean difference (SMD) is used to estimate the effect size. In the included studies, the serum IL33 were measured in ELISA with different source reagent, and the differences in the mean levels of IL33 were considered significant, therefore, SMD in serum IL33 was used to estimate the effect size. Heterogeneity was assessed using a chi-squared Q test and I-squared statistics. If PQ<0.1 or I2>50%, the heterogeneity was considered significant, and a random-effects model was used.
ResultsSearch resultsThe steps for screening and the study selection procedure are presented in Fig. 1. A total of 355 relevant articles were initially identified from PubMed, Embase, the Cochrane Library and other Chinese Medical Databases. Through screening of titles and abstracts, nine papers met our inclusion criteria screening, published between 2013 and 2016. We excluded 346 irrelevant or duplicate articles.
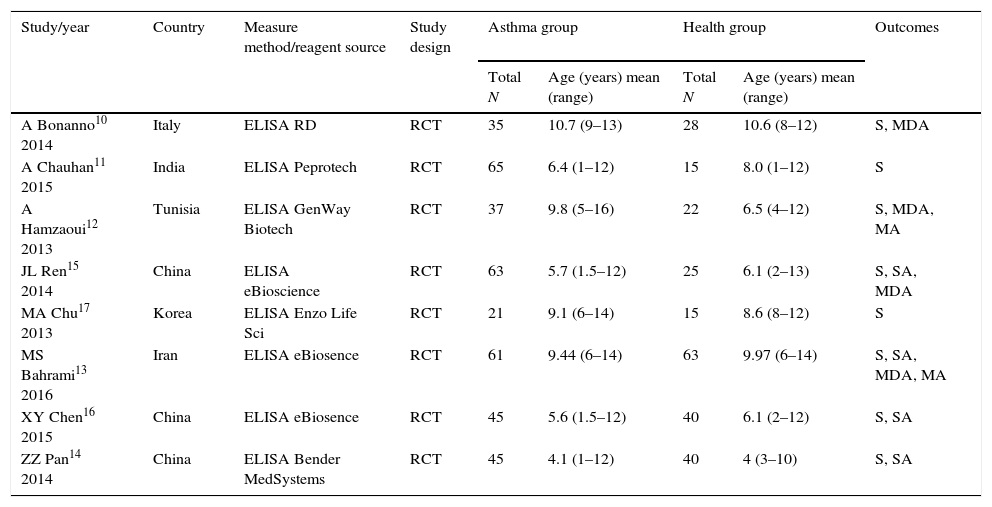
Quality and characteristics of included studiesIn this meta-analysis, according to QUADAS, one of the reports (n=9 studies) was classified as low quality, and the other eight studies were considered high quality. The low-quality study was excluded from the meta-analysis. The main characteristics of the included studies are summarized in Table 1. Meanwhile, the QUADAS result about the level of risk of bias for each included study was shown in Fig. 2. A total of 330 asthma children and 248 healthy children were included for data synthesis. Asthma diagnosis and assessment of severity were performed according to Global Initiative for Asthma (GINA) guidelines. Enzyme linked immunosorbent assay (ELISA) was adopted in all the included studies.
Characteristics of studies included in the meta-analysis.
| Study/year | Country | Measure method/reagent source | Study design | Asthma group | Health group | Outcomes | ||
|---|---|---|---|---|---|---|---|---|
| Total N | Age (years) mean (range) | Total N | Age (years) mean (range) | |||||
| A Bonanno10 2014 | Italy | ELISA RD | RCT | 35 | 10.7 (9–13) | 28 | 10.6 (8–12) | S, MDA |
| A Chauhan11 2015 | India | ELISA Peprotech | RCT | 65 | 6.4 (1–12) | 15 | 8.0 (1–12) | S |
| A Hamzaoui12 2013 | Tunisia | ELISA GenWay Biotech | RCT | 37 | 9.8 (5–16) | 22 | 6.5 (4–12) | S, MDA, MA |
| JL Ren15 2014 | China | ELISA eBioscience | RCT | 63 | 5.7 (1.5–12) | 25 | 6.1 (2–13) | S, SA, MDA |
| MA Chu17 2013 | Korea | ELISA Enzo Life Sci | RCT | 21 | 9.1 (6–14) | 15 | 8.6 (8–12) | S |
| MS Bahrami13 2016 | Iran | ELISA eBiosence | RCT | 61 | 9.44 (6–14) | 63 | 9.97 (6–14) | S, SA, MDA, MA |
| XY Chen16 2015 | China | ELISA eBiosence | RCT | 45 | 5.6 (1.5–12) | 40 | 6.1 (2–12) | S, SA |
| ZZ Pan14 2014 | China | ELISA Bender MedSystems | RCT | 45 | 4.1 (1–12) | 40 | 4 (3–10) | S, SA |
S: serum; SA: severe asthma; MDA: moderate asthma; MA: mild asthma; RCT: randomized controlled trial.
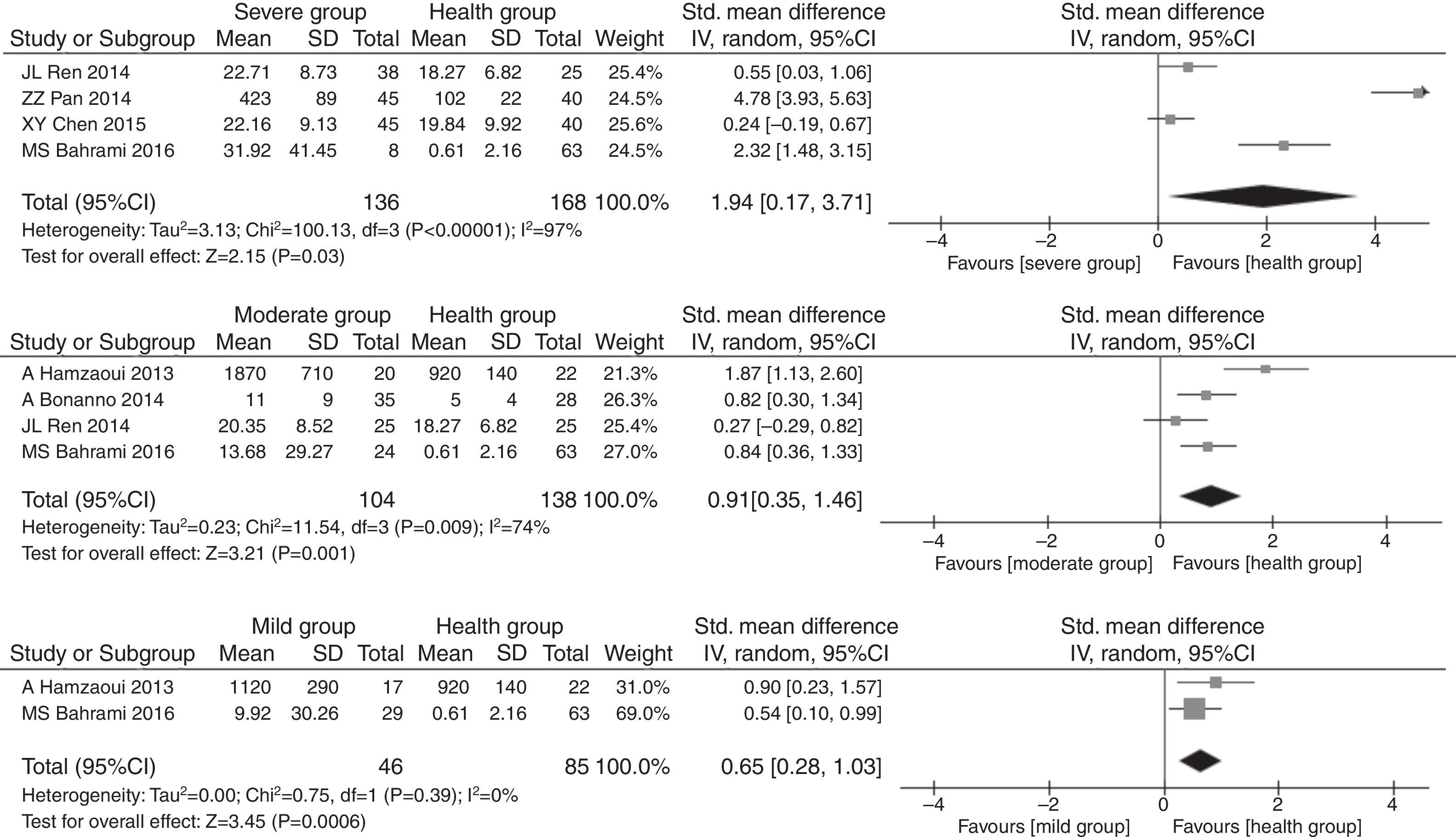
The pooled SMDs revealed that serum IL33 levels were significantly higher in the childhood asthma group as compared to the age-match health control group (SMD=1.32, 95% CI: 0.54–2.10, P=0.0009), as given in Fig. 3. Substantial heterogeneity was observed (I2=94%; P<0.00001) among studies. In order to minimize heterogeneity among the included studies, we compared serum IL33 level in different asthma progresses sub-group to health control group. The severe (involving four documents, SMD=1.94, 95% CI: 0.17–3.71, P=0.03), moderate (involving four documents, SMD=0.91, 95% CI: 0.35–1.46, P=0.001) or mild (involving two documents, SMD=0.65, 95% CI: 0.28–1.03, P=0.0006) asthma group had higher serum IL33 level. The heterogeneity of severe (I2=97%, P<0.00001), moderate (I2=74%, P=0.009) or mild (I2=0%, P=0.93) asthma group were observed, respectively (Fig. 4).
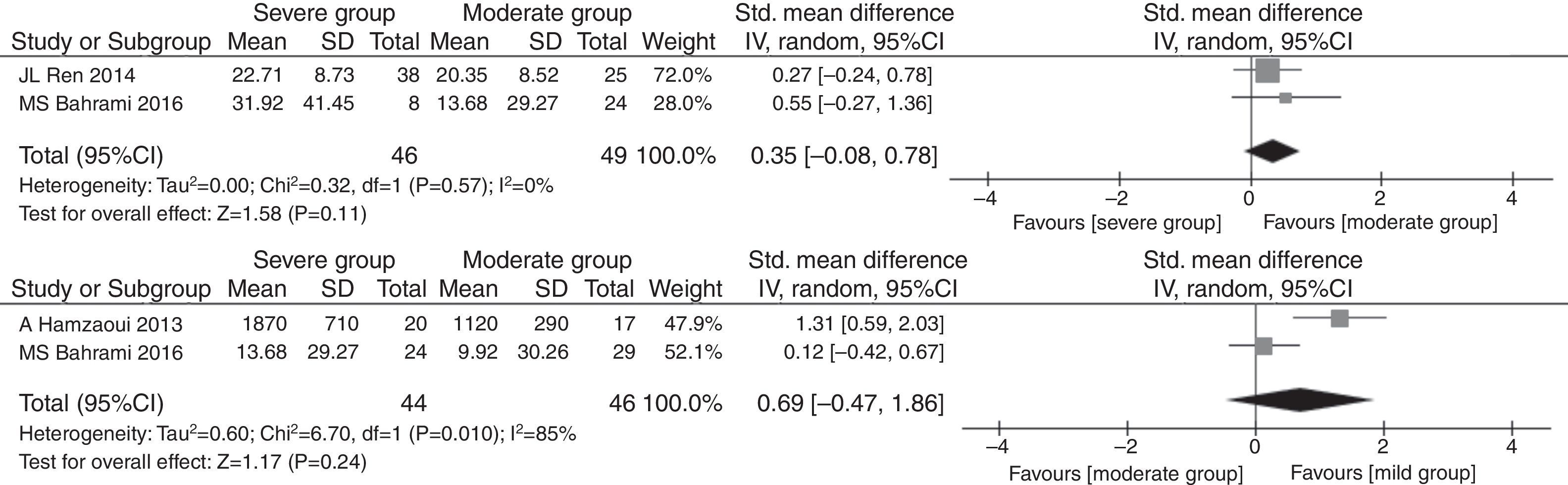
As described above, there were significant differences between serum IL33 level in asthma children and healthy children. However, the IL33 changes in different childhood asthma progresses are unknown. As shown in Fig. 1, severe asthma and mild asthma are reported at the same time by only one study. So we only evaluated the serum IL33 level between severe vs. moderate (SMD=0.35, 95% CI: −0.08 to 0.78, P=0.11) and moderate vs. mild (SMD=0.69, 95% CI: −0.47 to 1.86, P=0.24), respectively. The heterogeneity of two asthma progress ((I2=0%, P=0.57) severe vs. moderate; (I2=85%, P=0.01) moderate vs. mild) were observed, respectively. The meta-analysis results show that there were no significant differences among two asthma progress in Fig. 5.
DiscussionIL33 is a recently found cytokine with multivalent functions. As described an alarmin, IL33 may serve as a biomarker associated with some infectious diseases. However, there is no systematic review and meta-analysis on the serum IL33 levels for diagnosis of childhood asthma. Therefore, we conducted this study to assess the validity of serum IL33 test for early diagnosis childhood asthma. In this meta-analysis, the serum IL33 levels were significantly higher in children with asthma compared to age-matched healthy controls.
Meanwhile, significant heterogeneity was observed across the studies. This heterogeneity remained after the subgroup analysis, which indicated that the study population, measure reagents and other covariates might be responsible for it. Of the eight eligible studies, five were conducted in Asian populations, including China (3), Korea (1) and India (1); one was in an Iran population, one was in a Tunisian population, and one was in an Italian population. We did not obtain any studies from North and South America. As described in Table 1, the ELISA kits in included studies are purchased from six different manufacturers. So, the sensitivity of measure reagents was different among the included studies, which could cause heterogeneity. Furthermore, there were a series of factors influencing serum IL33 level in asthma patients, including year, sex and disease progress, which can cause heterogeneity.
Because IL-33 is a novel cytokine of the IL-1 family, we could not reach enough data to perform a statistical analysis. Severe asthma and mild asthma are reported at the same time by only one study. Thus, we could not obtain the statistic serum IL33 level between severe asthma and mild asthma. Two studies described the IL33 level between and among two asthma progress (severe vs. moderate; moderate vs. mild), respectively. Owing to the lack of enough data, there were no significant statistical differences among two asthma progresses.
In conclusion, although limited studies have reported serum IL33 level in childhood asthma, our meta-analysis shown serum IL33 level in childhood asthma is higher than that in healthy children. The finding may be useful for early diagnosis of childhood asthma.
Ethical disclosuresConfidentiality of dataThe authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
Protection of human subjects and animals in researchThe authors declare that no experiments were performed on humans or animals for this investigation.
FundingThere was no funding support.
Authors’ contributionsS Hua conceived and designed the experiments. Y Wang and L Wang performed the data extraction. Y Wang analyzed the data and wrote the paper.
Conflict of interestThe authors report no conflict of interest.
We thank all authors of primary studies included in our meta-analyses. There was no funding support.