Monoclonal anti-IgE antibody omalizumab is a promising therapeutic option in patients with chronic urticaria (CU) resistant to non-sedating H1-antihistamines (nsAH). However, data about its long-term efficacy and safety are still scant.
ObjectiveWe retrospectively analysed the clinical course of patients with severe recalcitrant CU that were treated in our department with omalizumab for a period greater than 24 months.
Methods and patientsSeven patients (six females, median 43 years) treated for a median of 35 months have been evaluated. Before treatment, all suffered from persistent symptoms despite receiving high doses of nsAH [4×/day], leukotriene antagonists and prednisolone (10–30mg/day for a median duration of 48 months). Response to treatment was assessed using urticaria activity score (UAS) and a combined symptom/medication score.
ResultsThere was a complete remission of disease in four patients after the first dose of omalizumab. Before the 5th administration, all patients had a UAS of 0. We found a significant improvement in UAS between pre-treatment and first dose (p=0.017) and a gradual decrease in the symptom/medication score over the course of the first five administrations. Tapering of prednisolone was possible in all patients. Administration intervals were gradually increased, although all experienced resurgence of symptoms in cycles greater than six weeks. There were no reported adverse reactions attributable to the drug.
ConclusionOmalizumab was a safe and effective corticosteroid alternative for maintaining long-term remission of symptoms in these patients. Treatment intervals required individual patient-by-patient determination. The drug did not seem to alter the natural history of the disease.
Urticaria is characterised by a transient, erythematous, pruritic wheal skin reaction that may occur with or without angio-oedema.1 Chronic urticaria (CU), defined by its persistence over six weeks, may occur spontaneously – chronic spontaneous urticaria (CSU) – or can be induced, either as a result of environmental physical stimuli, such as pressure, heat or cold (physical urticaria), or by other means (cholinergic, aquagenic, etc.). It is a relatively frequent condition and recent studies estimated that over five million patients in Europe alone suffer from persisting urticaria symptoms.1 Patients with CU are often severely impaired in their quality of life2 as the condition affects sleep, daily activities, professional life and social interaction.
The cause of CSU is unknown in approximately 50% of the cases,1 although studies during the last decade identified the presence of anti-IgE and/or anti-Fc¿RI IgG auto-antibodies in a subgroup of patients, suggesting a possible underlying autoimmune mechanism.3
Current European Academy of Allergy and Clinical Immunology (EAACI)/Global Allergy and Asthma European Network (GA2LEN)/European Dermatology Forum (EDF)/World Allergy Organization (WAO) guidelines state that the aim of treatment for all types of urticaria is to achieve complete symptom relief.4 Thus, identified underlying causes should be eradicated and, if necessary, symptomatic treatment should be established.
The first-line therapy for CU are the second-generation non-sedating oral H1-antihistamines (nsAH). Recalcitrant cases may be treated with up to fourfold increased doses of nsAH, with association with leukotriene receptor antagonists or by choosing a different nsAH. Association of H2-antihistamines, omalizumab or a variety of drugs with a relatively low level of evidence of efficacy constitute the last line of treatment for this condition.4
Omalizumab (Xolair®, Novartis, Switzerland) is a monoclonal humanised IgG antibody that attaches to unbound IgE's C¿3 domain, preventing its attachment with the high or low-affinity receptors (Fc¿RI and Fc¿RII) present in the membrane of mast cells, basophils, eosinophils and lymphocytes. Inflammatory mediator release is therefore reduced, as seems to be the surface expression of Fc¿RI receptors in basophils and mast cells.5 Omalizumab was approved in Europe in 2005 for the treatment of severe allergic asthma, initially in patients older than 12 years. Although the underlying mechanism in CU is assumed not to be of allergic origin, the assumption that auto-allergic mechanisms allowing mediator release in mast cells and basophils could be blocked, lead to the first publication of use of omalizumab in CSU in 2007, with favourable results.6 Since then, several publications including four randomised controlled multicentre trials6–9 have demonstrated that treatment with omalizumab seems to be safe and well tolerated. The majority of publications also seem to point out that a large improvement is usually evident after the first doses of treatment with further improvement in subsequent administrations. In the first published works, omalizumab dosage was calculated according to patients’ IgE levels and body weight as for severe asthma treatment. After 2011, authors mostly used a fixed dose of 300mg, identified as the possible optimal dose for the treatment of urticaria symptoms.8
Since 2009, our department has used omalizumab for the treatment of severe, treatment-resistant CSU. The objective of this work is to present data concerning the first seven CSU-patients included for omalizumab treatment, with a focus on the long-term prognosis of these patients.
Methods and patientsThis retrospective analysis examines the clinical data of seven patients with severe refractory CSU submitted to a treatment with omalizumab since March 2009. As most data are not normally distributed, values are expressed as medians and interquartile ranks. We used Mann–Whitney U test to compare non-normally distributed quantitative variables. Statistical data were analysed in SPSS software 12.0 (IBM Corporation, New York, USA).
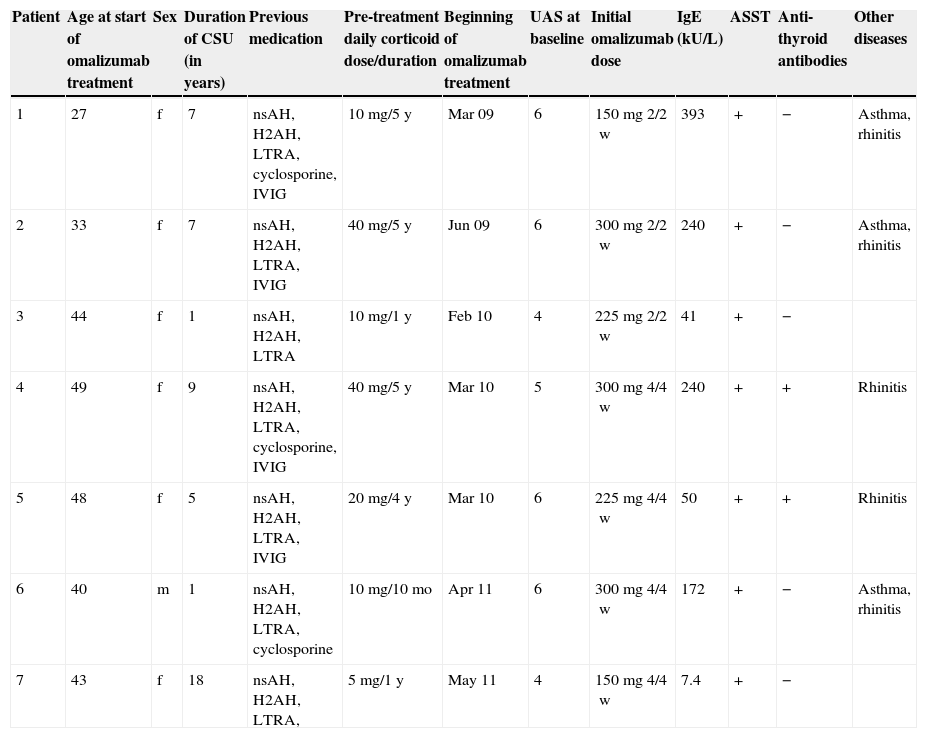
Patients (six females, one male) with a median age of 43 (36.5–46) years were evaluated according to the EAACI/GA2LEN/EDF/WAO guidelines and presented with CSU with at least three years of duration (median seven (3–8) years). The characteristics of the patients are presented in Table 1. All suffered from persistent symptoms despite receiving high doses of nsAH (4×/day), leukotriene antagonists and H2-antihistamines. All patients also previously required prednisolone to achieve symptomatic control. The median minimum dose required was 10 (10–30)mg/day for a median duration of 48 (12–60) months. All patients reported weight or mood changes that they associated with steroid therapy. Patients 1 and 2 had cushingoid facies.
Patient characteristics. CSU, chronic spontaneous urticaria; UAS, urticaria activity score; ASST, autologous serum skin test; nsAH, non-sedating H1-antihistamine; H2AH, H2-antihistamine; LTRA, leukotriene receptor antagonist; IVIG, intravenous immunoglobulin IgG.
| Patient | Age at start of omalizumab treatment | Sex | Duration of CSU (in years) | Previous medication | Pre-treatment daily corticoid dose/duration | Beginning of omalizumab treatment | UAS at baseline | Initial omalizumab dose | IgE (kU/L) | ASST | Anti-thyroid antibodies | Other diseases |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 27 | f | 7 | nsAH, H2AH, LTRA, cyclosporine, IVIG | 10mg/5y | Mar 09 | 6 | 150mg 2/2w | 393 | + | − | Asthma, rhinitis |
| 2 | 33 | f | 7 | nsAH, H2AH, LTRA, IVIG | 40mg/5y | Jun 09 | 6 | 300mg 2/2w | 240 | + | − | Asthma, rhinitis |
| 3 | 44 | f | 1 | nsAH, H2AH, LTRA | 10mg/1y | Feb 10 | 4 | 225mg 2/2w | 41 | + | − | |
| 4 | 49 | f | 9 | nsAH, H2AH, LTRA, cyclosporine, IVIG | 40mg/5y | Mar 10 | 5 | 300mg 4/4w | 240 | + | + | Rhinitis |
| 5 | 48 | f | 5 | nsAH, H2AH, LTRA, IVIG | 20mg/4y | Mar 10 | 6 | 225mg 4/4w | 50 | + | + | Rhinitis |
| 6 | 40 | m | 1 | nsAH, H2AH, LTRA, cyclosporine | 10mg/10mo | Apr 11 | 6 | 300mg 4/4w | 172 | + | − | Asthma, rhinitis |
| 7 | 43 | f | 18 | nsAH, H2AH, LTRA, | 5mg/1y | May 11 | 4 | 150mg 4/4w | 7.4 | + | − |
Additionally, four patients had previously been treated with immunoglobulin (400–1000mg/kg/month during 12–18 months) and three patients with cyclosporine (2–4mg/kg/daily during 6–18 months). Attempts to control the disease with those medications were either unsuccessful or led to unacceptable side-effects.
Three patients suffered from allergic asthma and rhinitis and two from allergic rhinitis only.
Clinical and laboratory examinations, including differential blood count, erythrocyte sedimentation rate, routine biochemistry and serological tests for autoimmune diseases did not reveal any underlying cause or illness, although all had positive autologous serum skin test (ASST) results and two had identifiable anti-thyroid auto-antibodies with normal thyroid function. Five patients had elevated total serum IgE (median: 206 [80.5–240]kUA/L).
Disease activity was evaluated using the urticaria activity score (UAS), a widely used patient-reported CSU measure with a simple scoring system, which captures intensity of pruritus and number of hives. Daily intensity of pruritus (range: 0 – none to 3 – severe) and number of hives ratings (range: 0 – none to 3 – more than 12 hives) are summed to create a daily UAS score (range: 0–6points/day).10 All patients presented with a pre-treatment UAS of 4 or greater.
After omalizumab treatment began, patients were weaned off corticosteroids as tolerated. Patients were instructed to use nsAH as rescue medication. For statistical analysis, we also used a combined symptom/medication score, as described in a previous study11 (0=no symptoms/no therapy; 1=no symptoms with nsAH on demand; 2=no symptoms with daily use of nsAH (up to fourfold); 3=recurrent symptoms under nsAH (updosing) in combination with leukotriene antagonists and/or H2-antihistamines; 4=no symptoms under immunosuppressive treatment (e.g. corticoid, dapsone and nsAH in up to fourfold dose, leukotriene antagonists and/or H2-antihistamines); 5=recurrent CSU symptoms under immunosuppressive treatment and nsAH in up to fourfold dose, leukotriene antagonists and/or H2-antihistamines). All patients presented with a pre-treatment symptom/medication score of 5.
Response to treatment was assessed based on the severity of clinical signs and use of concomitant medication before each injection (cycle) of omalizumab.
Consent from the ethics committee and hospital administration was obtained, as well as from all patients who signed an informed written consent.
As the patients began treatment with omalizumab before 2011, the dosage of the drug was calculated according to patients’ IgE levels and bodyweight as suggested for severe asthma treatment. As some patients were medicated with immunosuppressant or immunomodulatory drugs, these were interrupted four weeks prior to the first administration.
All administrations of omalizumab were made under medical surveillance in our daily care unit. The observation time was 2h in the first administration, 1h in the 2nd to 5th administrations and 30min thereafter. Patients were evaluated before each administration, which included a brief physical exam, UAS assessment and daily/rescue medication usage.
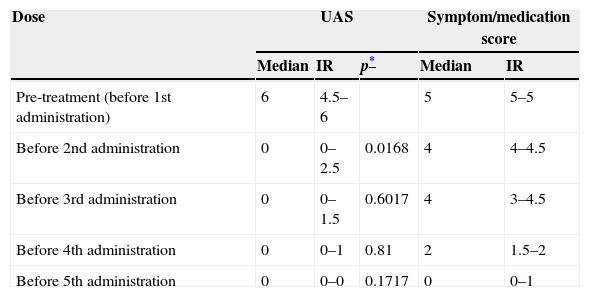
ResultsPatients have been treated with omalizumab for a median of 35 [29–40] months. There were no dropouts. Patients 1, 2, 4 and 7 (57%) achieved complete remission after the first dose of omalizumab, defined as a UAS of 0 within one week after the first administration. Before the 5th administration, all patients had a UAS of 0. The improvement seemed to be independent of age, sex, pre-treatment UAS, IgE level or presence of anti-thyroid antibodies.
We found a significant improvement in UAS between pre-treatment and first dose (p=0.017). Both variables – pruritus and number of hives – were significantly reduced.
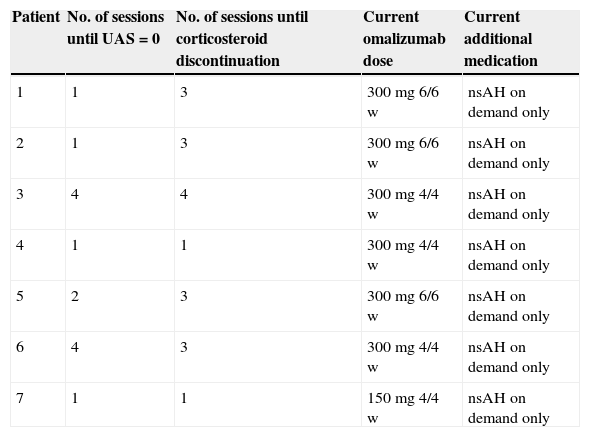
Treatment with omalizumab resulted in a substantial decrease in concomitant medication use and the symptom/medication score decreased gradually as shown in Tables 2 and 3. Tapering of prednisolone was possible in all patients. After four doses, only two patients required the use of any antihistamine; one patient took 10mg cetirizine per day, another 10mg levocetirizine per day. The cushingoid appearance of patients 1 and 2 disappeared three months after discontinuation of prednisolone.
Patient evolution and current therapy. nsAH, non-sedating H1-antihistamine.
| Patient | No. of sessions until UAS=0 | No. of sessions until corticosteroid discontinuation | Current omalizumab dose | Current additional medication |
|---|---|---|---|---|
| 1 | 1 | 3 | 300mg 6/6w | nsAH on demand only |
| 2 | 1 | 3 | 300mg 6/6w | nsAH on demand only |
| 3 | 4 | 4 | 300mg 4/4w | nsAH on demand only |
| 4 | 1 | 1 | 300mg 4/4w | nsAH on demand only |
| 5 | 2 | 3 | 300mg 6/6w | nsAH on demand only |
| 6 | 4 | 3 | 300mg 4/4w | nsAH on demand only |
| 7 | 1 | 1 | 150mg 4/4w | nsAH on demand only |
Evolution of UAS and symptom/medication score before each administration.
| Dose | UAS | Symptom/medication score | |||
|---|---|---|---|---|---|
| Median | IR | p* | Median | IR | |
| Pre-treatment (before 1st administration) | 6 | 4.5–6 | 5 | 5–5 | |
| Before 2nd administration | 0 | 0–2.5 | 0.0168 | 4 | 4–4.5 |
| Before 3rd administration | 0 | 0–1.5 | 0.6017 | 4 | 3–4.5 |
| Before 4th administration | 0 | 0–1 | 0.81 | 2 | 1.5–2 |
| Before 5th administration | 0 | 0–0 | 0.1717 | 0 | 0–1 |
No patients reported any adverse effect during the treatment.
After publication of the possible optimal dose for the treatment,8 patients with bimonthly administrations were changed to 300mg/monthly with no clinical repercussions.
After 12 months of satisfactory control of urticaria, an increase in the period between each administration was attempted on patient 1. As she remained asymptomatic with five and afterwards six weeks between treatments, omalizumab was suspended. Approximately six weeks after the last administration of omalizumab, the patient began experiencing symptoms of urticaria. An attempt was made to control the symptoms with high doses of nsAH, leukotriene antagonists and H2-antihistamines but, as previously, her CSU proved unresponsive. Subsequently, omalizumab was reintroduced with an excellent response to the first administration. Further attempts after 18 months of treatment in patient 2 and after 24 months in patient 5 were made with similar results.
Patients 1, 2 and 5 receive administrations every six weeks, whereas all others need monthly doses to remain symptom free. Safety and efficacy were maintained throughout the treatment.
DiscussionCSU is a disease that causes significant morbidity, often on a daily basis. At this time, the treatment of CSU with medications other than nsAH is not well standardised and often based on individual experience.4,12,13 We report herein a very good short and long-term response to omalizumab in severe patients with CSU who were unresponsive to high doses of nsAH, corticosteroids and, in some cases, cyclosporine and intravenous immunoglobulin G.
All patients responded and reported rapid relief of hives and pruritus, sometimes within 48h after omalizumab injection. Other studies also report such prompt results5,7–9,11 although the subjacent mechanism is still unknown. In previous studies that measured FceRI kinetics after omalizumab treatment14,15 significant downregulation of receptor numbers took at least several days. It seems unlikely that omalizumab could produce a decrease in IgE receptor density in an even shorter interval. Thus rapid reduction of plasma IgE levels might non-specifically downregulate responsiveness of cutaneous mast cells and/or circulating basophils by mechanisms not yet determined that are independent of surface IgE receptor density.
The mechanism with which omalizumab induces long-term remission is also unclear. This study seems to indicate that although patients with CSU benefit from this treatment, most may require ongoing maintenance dosing. In contrast to another study that focused at the long-term efficacy of this therapy,11 all patients in our group needed regular omalizumab administration to remain symptom-free. Two phase III multicentre trials with follow-up periods of 16 weeks have also demonstrated that an average of 10 weeks after omalizumab discontinuation, patients’ symptom scores were again similar to their baseline.8,9 These data lead us to speculate that omalizumab does not alter the natural history of CSU and that remission of its symptoms would occur regardless of omalizumab administration.
Notwithstanding, based on our retrospective data, omalizumab was an effective and safe alternative in maintaining long-term remission in patients with severe CSU. As the optimal duration of therapy and dosing interval have not yet been studied, we propose that patients be assessed on an individual basis as certain patients seem to be able to tolerate dose intervals higher than four weeks.11,16
Patients in our study were previously dependent on relatively high doses of prednisone to function without disabling symptoms. What is considered to be the main benefit of omalizumab therapy in patients with severe refractory CSU is that alternative agents are associated with significant adverse effects when used in the long term. In fact, weight or mood changes and cushingoid appearance were present in some of our patients before treatment and successful discontinuation of prednisone allowed a reversal of those conditions. No adverse reactions were reported in over 500 administrations of omalizumab in these patients. Other studies have confirmed omalizumab's safety profile in periods of 24 weeks; however, there are still few data concerning its safety in longer treatments. Currently, our patients have received omalizumab administrations for a median of 35 months without any reported adverse reactions attributable to the drug.
Because of the small patient population in our study, it is difficult to determine any patient characteristics that are predictors of response to omalizumab treatment. The presence of thyroid autoantibodies, total IgE, sex, and age did not appear to predict response. Although all patients had a positive ASST, other works also demonstrated its efficacy in patients without demonstrable auto-reactivity.17
In conclusion, omalizumab seems to be a safe, effective, steroid-sparing therapy for many patients with severe CSU who have been refractory to other treatments. Its onset of action is rapid and does not seem to lose its efficacy as far as 40 months after initiation of therapy.
This outcome, apart from offering a promising treatment for a highly disabling disease, offers new approaches to the study of CSU pathophysiology, given the multiplicity of effects of omalizumab therapy. Large clinical trials will be needed to establish its indication as a therapy for chronic urticaria, particularly when it is resistant to antihistamines, and to determine the best protocol for drug administration.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Confidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Conflict of interestAll authors consider that there is no financial or personal relationship which could result in a conflict of interest with regard to the published article.