Methacholine challenge test (MCT) performed with spirometry is a commonly used test to evaluate bronchial hyperreactivity (BHR) in children. However, preschoolers do not usually collaborate.
ObjectivesTo assess the usefulness of MCT through clinical evaluation (wheezing auscultation and decreased pulse arterial oxygen saturation [SpO2]) in recurrent wheezing preschoolers with asthma, in comparison to healthy controls.
MethodsWe performed the MCT (modified Cockroft method) on healthy and on asthmatic preschoolers. The end point was determined by the presence of wheezing in the chest and/or tracheal auscultation (PCw) and/or a decrease in SpO2 of ≥5 from the baseline value (PCSpO2). Maximal methacholine concentration was 8mg/ml.
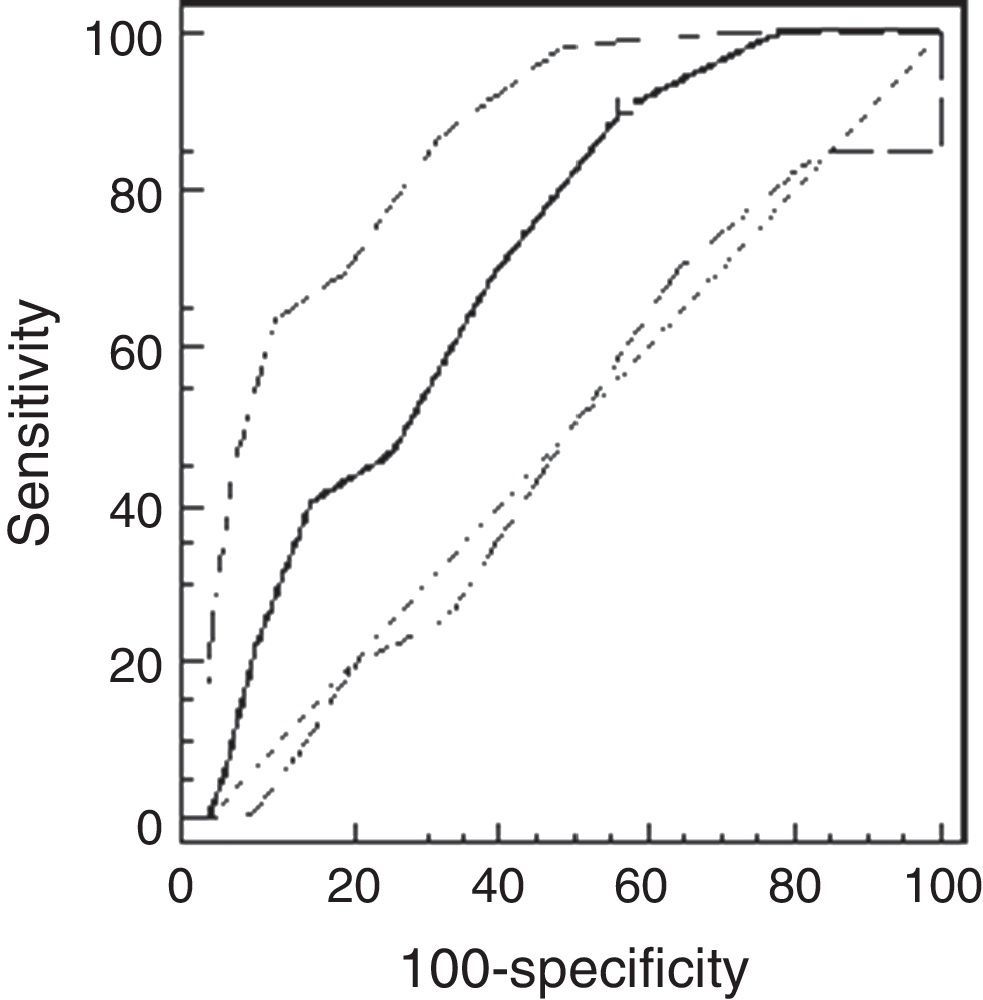
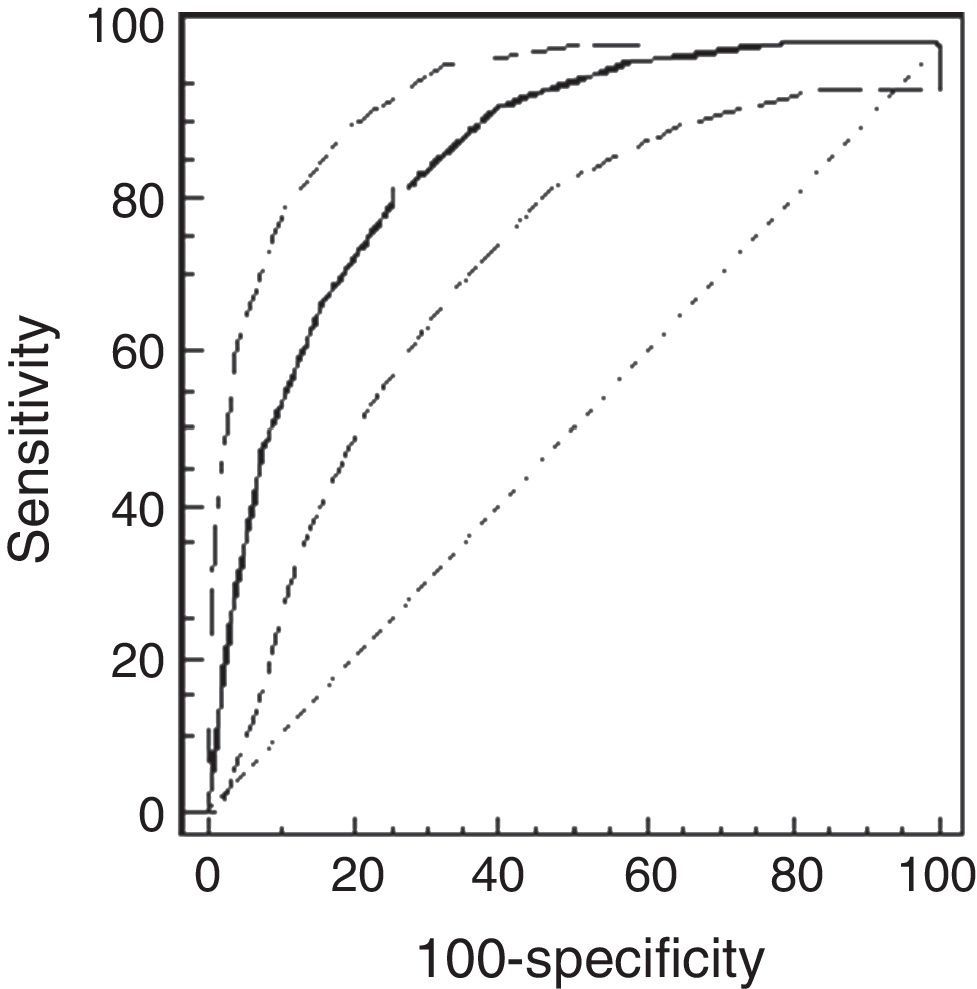
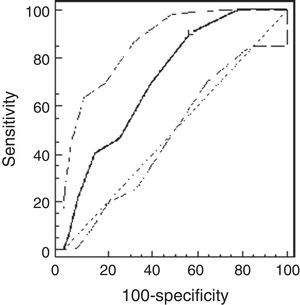
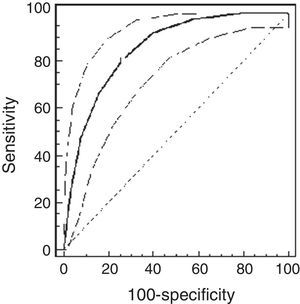
ResultsThe study population comprised 65 children: 32 healthy and 33 asthmatic children. There were no differences in demographic characteristics between the groups. The median methacholine doses for PCw and for PCSpO2 were significantly lower among asthmatic than healthy children: 0.5mg/ml (0.25–0.5mg/ml) vs. 2mg/ml (1–4mg/ml), respectively, p<0.001; and 0.25mg/ml (0.25–0.5mg/ml) and 2mg/ml (0.5–4mg/ml), respectively, p<0.001. The best cut-off point of PCw was observed at a methacholine concentration of 0.5mg/ml (AUC=0.72 [95% CI=0.66–0.77]), its sensitivity was 91%, specificity 43%, PPV 16% and NPV 98%. For PCSpO2 the best cut-off point was a methacholine concentration of 1mg/ml (AUC=0.85 [95% CI 0.81–0.89]), with sensitivity of 80%, specificity 74%, PPV 49%, and NPV 92%. There were no adverse reactions.
ConclusionMCT using clinical parameters such as wheezing auscultation and SpO2 measurement could be a useful and safe test to confirm BHR among preschoolers.
Asthma is the most prevalent chronic disease among children and in the vast majority of cases it starts at preschool age.1,2 However, it continues to be a difficult disorder to diagnose in preschoolers. This is partly because clinical symptoms of asthma are variable and non-specific, given that other wheezing disorders exist.3,4 Diagnosis and management of asthma in recurrent wheezing preschoolers are still primarily based on subjective clinical features and findings from medical examinations (atopic manifestations, parental asthma, or response to controller therapy [e.g. inhaled corticosteroids]).3,4
On the other hand, bronchial hyperreactivity (BHR) is a traditional hallmark of asthma. Its presence is a good predictor of severity, morbidity, and decline of lung function among asthmatic children.5,6 Due to its high sensitivity, methacholine challenge test (MCT) performed with spirometry is one of the most common tests for measuring BHR in schoolchildren and adolescents.7 Nevertheless, since preschoolers usually collaborate poorly in performing acceptable and reproducible serial spirometry manoeuvres, methacholine challenge tests with measurements that require little cooperation, e.g. impulse oscillometry, interrupter technique, and simpler clinical methods such as wheezing auscultation and pulse oximetry saturation [SpO2], have been developed.8–12
The aim of this study was to assess the usefulness and safety of a clinical method for measuring methacholine bronchial hyperresponsiveness through wheezing auscultation, decreased pulse arterial oxygen saturation [SpO2] and respiratory rate [RR] in recurrently wheezing preschoolers with asthma and healthy controls. The second aim was to establish methacholine concentration at which a significant airway response occurs.
MethodsThis study was carried out at the Pontificia Universidad Catolica de Chile, Santiago, Chile. We prospectively enrolled preschool children related to university employees, outpatients of the paediatric clinic, and children recruited from kindergartens in Santiago. The children were classified as asthmatics or healthy (control group). Asthmatics were included in the study if they had had three or more wheezing episodes in the last 12 months and if they had shown a clinical response to bronchodilator and to controller drugs (e.g. inhaled corticosteroids [ICS] or leukotriene inhibitors).13 They were classified by their severity, according to guidelines, as having intermittent, mild or moderate persistent asthma.14 None of the children had a personal history of prematurity, neonatal lung disease, pneumonia, lung resection, central airway obstructive disease or other cardiopulmonary chronic diseases.
The MCT was performed in our paediatric lung function laboratory during the summer of 2008–2010. Neither asthmatic nor healthy children had had any upper or lower respiratory symptoms for at least three weeks prior to the study, nor active rhinitis symptoms. Asthmatics had been free of controller drugs ICS and leukotriene inhibitors for at least one month prior to the study, anti-histamines for at least one week, and short acting bronchodilators for at least 8h. Children were seated with one parent in a non-stressful environment. The MCT was performed using the 2-min tidal breathing method developed by Cockroft et al.12,15 with doubling doses of methacholine solutions from 0.06 to 8mg/ml dissolved in saline. The children were previously nebulised with saline to establish control values. We used a nebuliser and a facemask (Pari Star®, Midlothian, USA). Sixty seconds after the end of each nebulisation, two independent observers (SC and RB or RR) simultaneously determined the respiratory rate for 1min, observed SpO2 in the monitor, and auscultated the presence of wheezing (over the trachea, and upper front and lower back of the thorax), asking the child to breathe deeper than tidal volume. SpO2 was monitored with a pulse oximeter (Masimo Rad 9®, Masimo Corporation, Irvine, CA, USA). Nebulisations were carried out every 5min until a maximum of 8mg/ml of methacholine; nebulisers were calibrated following ATS recommendations,15 with an output of 0.13ml/min ±10%.
The endpoint of MCT was set with the concentration of methacholine that determined one or more of the following events: presence of wheezing at auscultation (PCw), and/or decrease of ≥5 from control SpO2 for at least 10s (PCSpO2) and/or increase in RR ≥50% from control RR (PCRR). If more than one event was present, i.e. presence of PCw and PCSpO2, we called it PCw-SpO2. Once the test was completed, children were nebulised with 0.25mg of ipratropium bromide+0.5mg fenoterol bromide (Berodual®, Boehringer Ingelheim, Rhein, Germany) with oxygen flow of 6l/min for 10min. The MCT was stopped and considered a failure if the child was uncooperative (cried, hyperventilated, spoke during nebulisation or removed the facemask) or if adverse effects appeared (tearing, nasal symptoms, headache) or if parents requested the test to be stopped.
All parents signed informed consent forms to authorise the participation of their children in the study. The Ethics Committee of the Medical Research Center of the School of Medicine of the Pontificia Universidad Catolica de Chile approved the study (CE #0016/08).
Statistical analysisTo calculate the sample size, we used a previous study16 in which we found a median of PCw among asthmatic preschoolers of 0.25mg/ml (0.06–4mg/ml) and 1mg/ml (0.5–8mg/ml) among healthy children. Therefore, to find significant differences between the two groups we needed at least 10 children in each group (with 80% power and 95% of significance level). We used the Student t-test for comparative analysis of the general characteristics of the groups. PCw and PCSpO2 were not normally distributed. Their results were expressed as median and interquartile range. We also obtained their logarithmic values (not shown). Mann–Whitney and/or Kruskal–Wallis test was used to compare the median of methacholine concentrations between the groups. A ROC curve analysis was performed to determine the cut-off points of wheezing auscultation and SpO2 fall. We calculated the AUC (area under curve). Sensitivity, specificity, and positive (PPV) and negative predictive values (NPV) with their 95% CI for PCw and PCSpO2 were additionally calculated. Two-tailed p values of ≤0.05 were considered significant.
SPSS v 15.0 statistical software package (IBM, Armonk, NY, USA) was used for the analysis.
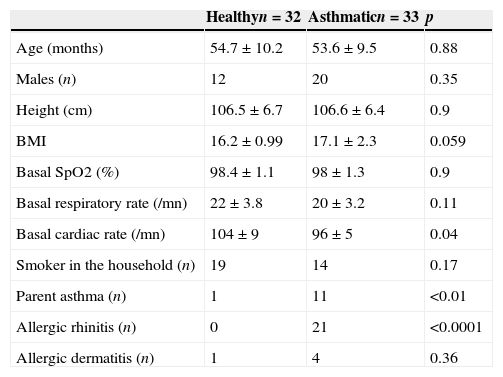
ResultsWe recruited 35 healthy and 37 asthmatic preschoolers. Seven preschoolers (three healthy) were excluded due to lack of collaboration to perform the MCT. There were no significant differences between healthy and asthmatic children in terms of age, gender, body mass index (BMI) and tobacco consumption at home. Basal SpO2 and RR were also similar between both groups, but cardiac rate was significantly higher in healthy children (Table 1). Asthmatic children had higher prevalence of dermatitis and rhinitis and parental asthma.
Demographic and clinical characteristics of healthy and asthmatic children.
| Healthyn=32 | Asthmaticn=33 | p | |
|---|---|---|---|
| Age (months) | 54.7±10.2 | 53.6±9.5 | 0.88 |
| Males (n) | 12 | 20 | 0.35 |
| Height (cm) | 106.5±6.7 | 106.6±6.4 | 0.9 |
| BMI | 16.2±0.99 | 17.1±2.3 | 0.059 |
| Basal SpO2 (%) | 98.4±1.1 | 98±1.3 | 0.9 |
| Basal respiratory rate (/mn) | 22±3.8 | 20±3.2 | 0.11 |
| Basal cardiac rate (/mn) | 104±9 | 96±5 | 0.04 |
| Smoker in the household (n) | 19 | 14 | 0.17 |
| Parent asthma (n) | 1 | 11 | <0.01 |
| Allergic rhinitis (n) | 0 | 21 | <0.0001 |
| Allergic dermatitis (n) | 1 | 4 | 0.36 |
Numbers are expressed as n or mean±SD.
Among asthmatics, 17/33 (52%) had intermittent symptoms, 14/33 (42%) had mild persistent symptoms and 2/33 (6%) had moderate persistent symptoms. For analysis, these last two children were included in the “mild persistent group”. Among asthmatics, the average onset age of wheezing was eight months and the average number of annual wheezing episodes was three.
The agreement between the two independent observers was 94% (34/36) for wheezing and 100% for SpO2. However, the measurement of RR (performed for 1min) was difficult and there was a high degree of discrepancy (42%) between the observers. Therefore RR was not considered for analysis. Considering the minimal doses to obtain one of the two clinical parameters (PCw or PCSpO2) as positive, there was no difference between asthmatics with intermittent and persistent symptoms: 0.38mg/ml (0.16–0.89mg/ml) and 0.54mg/ml (0.26–1.12mg/ml) respectively for PCw and 0.59mg/ml (0.06–5.78mg/ml) and 0.25mg/ml (0.14–1.46mg/ml) respectively for PCSpO2. Consequently, we joined the two sub-groups of asthmatics and compared it to the healthy control group.
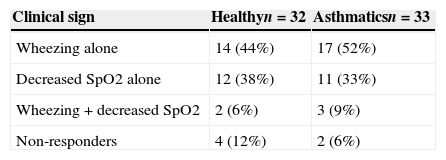
The maximal methacholine dose for asthmatic and healthy children was 8mg/ml and the lowest SpO2 was 90%. There were two children in the asthmatic group and four in the healthy group who did not show any positive test (Table 2). PCw did not show differences by age nor gender in both asthmatic and control group. The same observation was found for PCSpO2.
Clinical signs at the endpoint of MCT in healthy and asthmatic children.a
| Clinical sign | Healthyn=32 | Asthmaticsn=33 |
|---|---|---|
| Wheezing alone | 14 (44%) | 17 (52%) |
| Decreased SpO2 alone | 12 (38%) | 11 (33%) |
| Wheezing+decreased SpO2 | 2 (6%) | 3 (9%) |
| Non-responders | 4 (12%) | 2 (6%) |
Methacholine dose for PCw was significantly lower among the asthmatic than healthy children: 0.5mg/ml (0.25–0.5mg/ml) vs. 2mg/ml (1–4mg/ml), respectively, p<0.001. Similarly, the methacholine dose for PCSpO2 was lower among the asthmatics than the controls: 0.25mg/ml (0.25–0.5mg/ml) and 2mg/ml (0.5–4mg/ml), respectively, p<0.001. Only four asthmatic children and two healthy children had a positive PCw and PCSpO2 test (Table 2), so we could not draw any valid conclusion combining both variables.
Using the ROC analysis, the best cut-off point of PCw was observed at a methacholine concentration of 0.5mg/ml (AUC=0.72 [95% CI=0.66–0.77]). Its sensitivity was 91% [95% CI=75–98%], specificity 43% [37–49%], PPV 16% and NPV 98% (Fig. 1). For PCSpO2 the best cut-off point was at a methacholine concentration of 1mg/ml (AUC=0.85 [95% CI 0.81–0.89]), sensitivity 80% [70–88%], specificity 74% [68–79%], PPV 49%, and NPV 92% (Fig. 2).
In the asthmatic group, there were 21 children with allergic rhinitis and 12 without rhinitis. However, there were no differences in PCw median between those with rhinitis and those without rhinitis: 0.25mg/ml (0.25–0.5mg/ml) and 0.5mg/ml (0.5–2mg/ml), respectively, p=0.37.
There were no adverse reactions in any children.
DiscussionIn the present study, we found that preschool children with intermittent and mild persistent asthma had a significantly lower methacholine dose for PCw and PCSpO2 than healthy controls. The best cut-off point of PCw was observed at a methacholine concentration of 0.5mg/ml and of PCSpO2 at 1mg/ml, both with good sensitivity and NPV. We report the term PCw as an analogy to PC20, as has been previously proposed.7 Besides, we added the PCSpO2 also as an analogy to PC20. This study shows that measuring methacholine induced BHR using PCw and PCSpO2 among preschool children with intermittent and mild persistent asthma is a useful, simple and low risk method.
Wheezes are produced by the fluttering of the airways walls and fluid together, and are induced by a critical airflow velocity.17 It must be considered that the conditions for hearing wheezing may change from breath to breath depending on the intensity of inhalation or exhalation or the presence of airway secretions.18 Optimal evaluation of wheezing is now available through devices that record and analyse breathing sounds, thus improving sensitivity and objectivity.19,20 In this study we asked children to breathe deeper than tidal volume to ensure a better breath sound auscultation. To avoid having a biased observation, both observers auscultated simultaneously, and they agree in 94%. We evaluated children 1min after nebulisation was complete. We did not hear breath sounds during nebulisation, as Godfrey et al. did.21 They assessed BHR to adenosine 5′-monophosphate in asthmatic preschoolers with acoustic analysis of both lungs and found that wheezing would start during nebulisation in 31% of children and that the site, timing and characteristics of the first wheeze showed high variability. Moreover, in a study in preschool children, Bentur et al.22 found that obtaining the standardised FEV1 (Forced Expiratory Volume in one second) fall of 20% by spirometry (PC20-FEV1) frequently preceded wheezing auscultation. This suggested that PC20-FEV1 determinations must be safer than the method used in our study. However, we did not have any adverse reactions and no child showed a fall of SpO2 below 90%.
SpO2 measurement is an indirect sign of bronchoconstriction, reflecting hypoxaemia caused by ventilation/perfusion alteration due to smooth muscle contraction, vasodilatation or both.23 We must consider that baseline SpO2 values correspond to the upper part of the oxyhaemoglobin saturation curve. So small changes in SpO2 could be associated with large drops in arterial oxygenation.24 Some researchers had used SpO2<90–91% or a 3–5 decrease as the end-point.8–10,22 A fall in SpO2 has been reported to have a good correlation with transcutaneous oxygen pressure and respiratory resistance in MCT carried out in asthmatic infants and preschoolers.24,25 Although the measurement of SpO2 is simpler and more widely available, we found that it could be used to assess bronchial hyperresponsiveness, and if wheezing is not present we could consider a concentration of 1mg/ml for PCSpO2. This parameter could be used for safety and BHR diagnosis in younger children.
Our study shows a lack of coincidence between decreased SpO2 (PCSpO2) and the presence of wheezing (PCw); both parameters were positive only in four asthmatic and two healthy preschoolers. This issue was reported by different authors with opposing results. Yong et al.10 obtained wheezing in 78%, decreased SpO2 in 10% and both variables in only 12% of recurrently wheezing <4-year-old children. In a group of 146 young asthmatics, Springer et al.7 found the presence of wheezing alone in 6.8%, decreased SpO2 in 5.5% and both in 13.6%. Koh et al.11 found wheezing alone in 37%, had decreased SpO2 alone in 37%, and both parameters in 26% preschool asthmatic children. Kivastik et al.8 stopped the test due to wheezing in 27% of healthy, coughing and wheezer children, in 33% because of decreased SpO2, and in 35% due to both variables. Our discordance could be explained by the effect of bronchoconstriction on breathing patterns, with minute ventilation and respiratory rate variations.26
The differences in PCw and PCSpO2 values between healthy and asthmatic preschool children in our study are remarkable, with a fourfold value in the last group. We found PCw median of 2mg/ml and 0.5mg/ml and for PCSpO2 2mg/ml and 0.25mg/ml in healthy and asthmatic children, respectively. Our results were expressed as median because they were not normally distributed. Other researchers reported their results as geometric mean. De Mir et al.27 studied 16 healthy and 63 asthmatic children aged from six months to four years old; they found higher values than ours in both groups, considering wheezing and/or SpO2 decrease as end-points: 13.3±5.02mg/ml in healthy children and 5.8±3.9mg/ml in asthmatic children. Kivastik8 found a geometric mean of PCw of 2.88mg/ml in nine healthy children and 1.28mg/ml in 25 asthmatic children. In these studies they had a low number of healthy children, inhaled corticosteroids were permitted for use as usual in children with recurrent wheezing, and they used a different methodology to calculate PC of the total group. These facts could explain the differences from our results.
Regarding the severity of asthma, we did not find significant differences in PCw or PCSpO2, similar to the findings of Wang et al.25 In contrast, Avital et al.28 reported a close relationship between the degree of clinical BHR and the severity of symptoms using the tracheal auscultation technique. Perhaps our results can be explained by the presence of a low asthma severity in our patients. It is also known that age correlates with an increase in methacholine-induced BHR in asthmatic children,29 but in our study we only found a similar trend, without statistical differences.
This study has some limitations. First, we only recruited intermittent and mild persistent asthmatic children, so these results cannot be applied in more severe asthmatic categories. Second, since this study was not longitudinal, we cannot determine if the presence of BHR among preschool children with intermittent and mild persistent asthma could be a marker of illness persistence later in life.5,6 Although sensitivity and NPV have high values, specificity and PPV are limited especially for PCw. Thus, BHR could be present in a child without asthma. Then MCT by this method could be useful to rule out asthma, like in older children and adults.15 Third, considering the prolonged time of the MCT, it was refused by seven children (9.7%); we should have considered a shorter test to increase its level of acceptance, as other researchers did.8,27 Fourth, the combination PCw and PCSpO2 often lacked coincidence, and this could be the best reliable parameter to evaluate clinical bronchial responsiveness to methacholine; we could not carry out analysis because of the fair number of cases in this situation. We know that FEV1 measurement is the best and safest parameter for detecting airway obstruction, nevertheless the aim of our study was to find alternative measures for patients who fail to perform spirometry. However, a strength in this study is the fact that a significant sample size was achieved and even exceeded. We used a safe method and followed ATS guidelines12,15 in performing the MCT (being critical for comparing results in clinical practice). Neither adverse reactions nor severe decreases in SpO2 were observed. Finally, we confirmed that checking SpO2 during MCT should be mandatory, given the fact that a decrease suggests bronchial obstruction in children without detectable wheezing.
ConclusionAlthough wheeze auscultation and SpO2 cannot completely replace lung function tests to evaluate BHR, this clinical method confirms that BHR is present in almost all preschool patients with three or more annual wheezing episodes. It also provides additional information about the normal response to methacholine in healthy children. This could be a safe, useful and tolerable method to use in young children who are uncooperative with spirometry, or when laboratories lack the equipment necessary to evaluate methacholine induced bronchoconstriction in this age group.
Ethical disclosuresProtection of human subjects and animals in researchThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Confidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in this study.
Right to privacy and informed consentRight to privacy and informed consent. The authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Conflict of interestThe authors have no conflict of interest to declare.
The authors thank the children, their parents and the kindergarten teachers.