Tremendous progress in the ability to identify and test the function of microorganisms in recent years has led to a much better understanding of the role of environmental and host microbiome in the development of immune function, allergic sensitization and asthma. In this review, the most recent findings on the relationships between environmental microbiota, respiratory, intestinal microbiome, the consequences of early-life microbial exposure type and gut–lung microbial axis and the development of asthma and atopy are summarized. The current perspective on gut and airway microbiome manipulation for the primary prevention of allergic diseases and asthma is also discussed.
For many years, substantial attention has been given to microbes as disease-causing agents and to the methods of combatting them. Today we know that the human body is inhabited by 10 times more microorganisms than the number of cells that our body counts. They affect many different life processes, and their presence is the guarantor of homeostasis. The role of microbes in health and disease prevention has been one of the most explored research topics in medicine in recent years.
This is a consequence of tremendous progress in the ability to identify and test the function of microorganisms by genotyping via analyzing sequences of the highly conserved 16S bacterial RNA region and comparing them with the known sequences accumulated in databases.1 Owing to these new techniques, we have learned that traditional methods, such as microscopic examination and in vitro bacterial culture, are able to detect only a small part of the microbial population colonizing the human body. Bacteria make up 2% of our body weight, and the number of bacteria is ten times greater than the sum of all the cells of the human body. Bacterial flora populates almost all the niches of the human body and plays an immense role in the process of maturation and functioning of the immune system.2
The first attempt to comprehensively characterize the human–microbe genome was made in the “Human Microbiome Project” launched in 2007.3 Thousands of samples from 250 healthy adult volunteers were taken, and complete microbiology of the intestines, skin, mouth, respiratory and genitourinary system was sequenced. The effects of age, diet, medication, living environment and diseases on the composition of this microflora were examined.
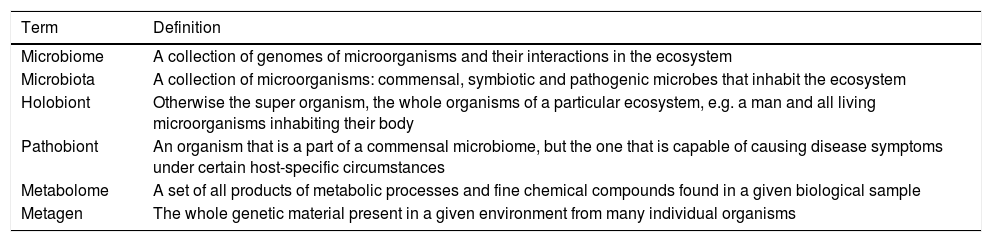
As a result of this developing microorganism research area, some concepts have been redefined and some new terms introduced (Table 1).2
Basic terms in microorganism research.
| Term | Definition |
|---|---|
| Microbiome | A collection of genomes of microorganisms and their interactions in the ecosystem |
| Microbiota | A collection of microorganisms: commensal, symbiotic and pathogenic microbes that inhabit the ecosystem |
| Holobiont | Otherwise the super organism, the whole organisms of a particular ecosystem, e.g. a man and all living microorganisms inhabiting their body |
| Pathobiont | An organism that is a part of a commensal microbiome, but the one that is capable of causing disease symptoms under certain host-specific circumstances |
| Metabolome | A set of all products of metabolic processes and fine chemical compounds found in a given biological sample |
| Metagen | The whole genetic material present in a given environment from many individual organisms |
For many years, it has been acknowledged that bacteria and viruses can aggravate the course of allergic diseases, such as bronchial asthma or atopic dermatitis. Now more and more we know about the positive role of microorganisms, both environmental and human body inhabited, in allergic diseases. The first reports on the role of environmental microbiome as a protective component came from the studies conducted in low prevalence allergy populations. It has been revealed that children living in Alpine farms, who had contact with farm animals and consumed unpasteurized milk, were less likely to have asthma, allergic rhinitis, and were less atopic than their peers from non-farming families.4 The high level of gram-negative bacterial endotoxins in samples of dust from the child's mattress correlated inversely not only with the prevalence of atopy measured by allergen-specific IgE level but also with bronchial asthma and cytokine production by peripheral blood leukocytes.5
The interesting research comes from Karelia, which is an area located on the Russian–Finnish border in the north-east of Europe. As a result of political decisions, the inhabitants of that area were separated by the country border after the Second World War. In the post-war decades the same ethnic group followed dramatically different lifestyles: on the Finnish side, very rapid westernization and modernization took place, making the Finnish Karelians a very high-civilized population. However, in Russian Karelia, the lifestyle did not change for decades with the model of a rural, low-income population, but with a very traditional style of life. In the 1990s and later in the 2000s, patients on both sides of the border were surveyed on the prevalence of atopy, allergic rhinitis, and bronchial asthma. In the Finnish area the prevalence of atopy, asthma and allergic rhinitis were one of the highest in Europe, while on the Russian side allergic diseases were practically absent, and, for example, the prevalence of birch pollen allergy was three times lower than on the Finnish side.6 Exploring the reasons for such large differences in the allergies prevalence, a number of studies have been conducted in these populations, and significant differences in exposure to microorganisms present in dust and drinking water and their association with atopy have been confirmed. On the Russian side, a significant microbial diversity was prevalent, and the bacterial count itself was significantly higher than in the environmental samples from Finnish Karelia. The bacteriological composition of the samples was also significantly different.7 Among healthy inhabitants of Finnish cities, the higher diversity of skin microbiota compared to atopic inhabitants and a strong association between the abundance of Acinetobacter on the skin and the expression of the anti-inflammatory cytokine IL-10 in peripheral blood mononuclear cells were found.6
Another example is the study of the Hutterites and the Amish, fairly similar religious communities living in North America and leading a rural lifestyle. The difference, however, is that the Amish do not accept any technical innovations, including in farming practices, they are called ‘detained in time’, while the Hutterites use all civilization facilities in their farm work. A recent study has shown a huge difference in the prevalence of allergic diseases among children in these two populations.8 Moreover, the level of bacterial endotoxin in the dust collected in the Amish houses was almost seven times higher than that in the Hutterites’ houses, and the bacteriological composition in the dust varied in both groups. In the experimental research, the Amish's dust exposure prevented the development of asthma in mice, but the dust from Hutterites’ houses induced bronchial hyperresponsiveness.9
The Gabriel project funded by European Commission aimed to identify the microbiological factors that prevent asthma in children in rural settings. The research was conducted in four countries: in southern Germany, Austria, Switzerland and in Poland in Lower Silesia. These were cross-sectional studies in the group of children aged 6–12 living in rural areas and in small towns. In the project, apart from the questionnaire survey, nasal bacteriological swabs, dust samples from a sleeping mattress, milk samples from a farm and barn dust samples were collected to carry out highly detailed microbiological analyzes. It has been documented that in the rural environment the microbial diversity and the number of bacteria and fungi are significantly higher.10 This specific microbial cocktail stimulates our immune system to protect against the development of allergies. The more bacteria and fungi in the environment the less likely bronchial asthma occurs. Recent studies from that population on microbiome detected in nasopharyngeal swabs have shown less biodiversity in nasal specimens and the presence of Moraxella among children with bronchial asthma not living in the farm households.11
The above studies, cross-sectional in their design, showed possible sources of environmental microbiome protection, but because of its nature were not able to disentangle the causal relationship and the role of exposure timeframe in these populations.
Prospective birth-cohort studies conducted in farm populations revealed that the early environmental exposures in the first months of life or even earlier in pregnancy, provided stronger protection than exposures occurring later in life and that critical period of life offers a unique opportunity of immunologic tolerance lesson, if the protective environmental exposures are present.12 Such an early “natural experiment” might be a good pattern for developing asthma and allergy-preventive therapeutic strategies.
Urban populationsIt is believed that farm living provides a richer and more diverse microbial environment than an urban one. Decreasing microbial diversity is associated with reduced contact with nature and predisposes to the development of allergic diseases. However, under certain conditions, not only a rural but also an urban environment can be a valuable source of microbial biodiversity. Interesting outcomes of research into the effects of exposure to microorganisms and allergens in early childhood were reported in the prospective URECA (Urban Environment and Childhood Asthma) study.13 In the survey conducted among children from large cities, but from their impoverished districts, in the United States, the bacterial composition of the household dust samples collected during the child's first year of life and the concentrations of cockroaches’, mice's and cats’ allergens were analyzed. The results indicated that the exposure to a higher level of bacterial and allergen biodiversity in that population reduced the risk of atopy and wheezing at the age of three. The lowest prevalence of atopy and wheezing occurred in children who were exposed to the highest levels of home pets’ allergens in their first year of life. It was recognized that the bacteria present in the environment could play the role as adjuvant inducing immune tolerance to allergens.13
Immunological effectsAll the above surveys provide the evidence that exposure to microorganism-rich and natural environment protects against allergy. Such exposure can have the ability to modify immune maturation in early life. The differences in pattern recognition receptors expression, in the activity of cytokines and function of regulatory T cells between farm and non-farm children were detected.14 Bacterial endotoxins present in farm dust modified the mechanisms of the primarily non-specific immune response to allergens. House dust mites induce activation of airway epithelial cells mediated by toll-like receptor 4 (TLR4). This process initiates activation of nuclear factor κB (NF-κB) and, as a consequence, secretion of pro-inflammatory mediators like chemokine CCL20 and granulocyte–macrophage–colony-stimulating factor (GM-CSF) which are necessary for the recruitment and maturation of dendritic cells. These cells mediate transport of inhaled allergen to regional lymph nodes, where priming and activation of Th2 cells, necessary for the production of IgE, takes place. This sequence of the inflammatory response can be suppressed by the exposure to bacterial lipopolysaccharides, as recently described by Schuijs et al.15 Airway exposure to endotoxins inhibited activation of NF-κB by the increase in the synthesis of its attenuator, enzyme A20. These associations, observed in an experimental model in mice, have been confirmed in further experiments on human bronchial epithelial cultures and in a case–control study of asthmatics. Enzyme A20 may be one of the potential therapeutic targets for asthma prevention, but it is clear that the mechanisms of environmental microbial exposure to allergy and asthma protection are much more complex and heterogenic. The diversity of bacterial and fungal species present in the farm environment, which exerted a protective role, suggests the involvement of multiple mechanisms.10 Moreover, interactions among viral respiratory tract infections, host response to environmental and commensals bacteria and atopy-associated inflammatory pathways were observed.16 Respiratory viruses weaken local defenses against opportunistic bacteria promoting pro-inflammatory responses and tissue damage and increase the risk of asthma.17 Microbiota can also influence the regulation of Treg (regulatory lymphocytes T), building a tolerance that protects against the onset of allergic reactions, but the exact mechanisms of interaction between altered innate immune response and adaptive immunity remain largely unknown.18
Recent findings showed that certain commensal bacteria in the human gut were able to produce active histamine, immune regulatory biogenic amine involved in an immediate type allergic reaction. Interestingly, the presence of a histamine-producing type of bacteria was increased in asthma patients.19
Human microbiome and its impact on allergy and asthmaEarly gut and lung colonizationAs previously described, it is believed that with the growth of civilization, hygiene improvement, and increasingly limited contact with nature we have lost the ability to stimulate our immune system by the environmental microbiome. But the changes have also influenced the composition of our human–microbe, which is the mediator between the environment and the immune system. Moreover, the interaction between the host's commensal and pathological microorganisms may be modulated in this context and may influence inflammatory pathways associated with allergy and asthma. Here, life's early stages seem to be crucial. Most studies emphasize the role of the so-called early microbiological programming – the impact that host-microbial components exert on both the innate and adaptive immune system during the first months and years of life.20,21 The very first contact with bacteria takes place in fetal life. The presence of bacterial DNA in the placenta, amniotic fluid and meconium has been confirmed. This intrauterine colonization of the fetus takes place both in healthy and high-risk pregnancies, although the type of bacteria detected may be influenced by maternal health factors and may have consequences for childhood health.22,23 The following colonization of the gastrointestinal tract takes place from the first days of life. In the first three years of life, intestinal biodiversity is formed, which, as shown by research, stabilizes in later life.2 Early studies in the 1990s showed differences in the composition of intestinal microflora of neonates in Estonia, a country with a low prevalence of allergic diseases and Sweden, with much higher allergy level.24 In the follow-up studies, the composition of intestinal bacteria in the first weeks of life influenced the risk of atopy at the age of two.25
Early bacterial colonization of the intestines, but also the airways and skin, may be regulated by various modifying factors that can affect the risk of future allergic diseases.26 The most important are: the use of antibiotics during pregnancy and the mode of delivery (during cesarean section the newborns are mainly colonized by bacteria from the mother's skin, in vaginal birth by vaginal bacteria), early antibiotic therapy in the newborn, feeding method (breastfeeding promotes colonization with bacteria that reduce the risk of allergies). Also, exposure to farm animals and unpasteurized milk consumption during pregnancy and infant period may be protective.27
Studies conducted in the group of healthy volunteers have shown that the lower respiratory tract is not sterile, bacteria early colonize the lungs. The bacterial lung composition reflects bacterial composition in the mouth and throat, but not in the nasal cavity. It is believed that the lungs are inhabited by the microbiome transported through micro-aspiration passages from the gastrointestinal tract and via inhalation.28
Impact on asthmaThe microbiological composition of respiratory tract differs between patients with asthma and healthy people. In well-controlled asthmatic patients, bacterial diversity was inversely proportional to bronchial hyperresponsiveness. The main bacterial strains isolated in patients with asthma were Proteobacteria; Bacteroides predominated in healthy individuals.29 Studies in patients with various phenotypes of asthma have also been conducted, and the presence of characteristic bacteria in particular asthma phenotypes has been confirmed (i.e., obesity-associated asthma, neutrophilic asthma, atopic asthma). Undoubtedly, dysbiosis occurs in asthmatics, but it is difficult to definitively determine the role and mechanisms of interdependence between bacterial composition and asthma phenotype. Studies were conducted in relatively small patient groups and require further confirmation.29 The answer to the question as to whether changes in the bacteriological composition in asthma are the cause or the consequence of this disease is mainly based on experimental animal studies. For example, exposure to allergens in germ-free mouse induced a severe allergic reaction, but after bacterial colonization, this strong reaction to allergen exposure was no longer present.30 The nasal exposure to farm dust resulting in specific nasal bacterial compositions (Acinetobacter lwoffi F78 and Lactococcus lactis G121) prevented airway inflammation both in exposed mice and their neonates.31
The PRACTALL document, presented by the American and European Academies of Allergy and Clinical Immunology, summarizes the current knowledge about the effects of particular respiratory and gastrointestinal bacteria on the risk of bronchial asthma.32 In cohort studies, the presence of Streptococcus, Haemophilus, and Moraxella in lower respiratory aspirates, but also in nasal swabs taken during the first month of life, increased the risk of bronchial asthma, wheezing and high IgE concentrations. When colonization occurred later – at 12 months of age, it no longer increased that risk.33 Thus, the type of early colonization seems to play a key role. But there is some microbiota, mainly colonizing the gut that can reduce the risk of bronchial asthma.32 This confirms the existence of the gut–lung axis. The lung microbial composition is affected by intestinal bacteria that are transported through micro-aspiration and bacterial metabolites circulating in the blood.
It has been shown that the type of bacterial colonization of gut at the first month of life may increase the prevalence of wheezing and asthma at five years of age. Moreover, the decreased intestinal microbiome diversity during the first weeks of life increases the risk of asthma.34 In the Copenhagen Prospective Studies on Asthma in Childhood2010 (COPSAC2010) birth cohort of 690 children followed from the birth up to five years of age, the nature of gut colonization patterns during the first year of life was associated with the later risk of asthma. Low microbial maturity, specific composition and diversity of the gut microbiome during the first year of life has an important role in the development of childhood asthma, especially in children born to asthmatic mothers. Moreover, having older siblings helped advance the maturation. These results suggest potential beneficial effects of specific microbial supplementation in the first year of life for children at high risk for developing asthma.35
Possibilities of interventionThe role of the microbiome in bronchial asthma requires further attention. It is a complex system of interconnections among environmental exposures, coexisting diseases, and interaction of the immune system with airways and gut microbiome. The role of specific bacteria species can vary depending on the phenotype of the disease and the way of treatment. Some bacteria can exacerbate the course of the disease, and others can be potentially used for therapeutic purposes. Microbiome manipulation has been explored by studying the use of probiotics and prebiotics. It is currently believed that different types of probiotics and prebiotics exert various immunological effects, but the results in asthma and other allergic diseases are unclear. Currently, the available evidence does not indicate that probiotic or prebiotic supplementation reduces the risk of developing an allergy in children.36 However, The World Allergy Organization (WAO) guideline, followed the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach, suggests using probiotics in specific groups like pregnant women at high risk for having an allergic child, in women who breastfeed infants at high risk of developing allergy and in such infants.37 For prebiotic supplementation, the group of not-exclusively breastfed infants was proposed.38 As authors of the WAO documents clearly stated, all these recommendations are conditional and supported by very low-quality evidence. Recently, the focus of attention has been given to the investigation of the immune response to nasal exposure to house dust mite allergens in mice with diets rich and poor in fermentable fibers.39 The results confirmed the differences in intestinal bacterial composition influenced by the diet and different immune responses. After the high-fermentable fibers diet, the bacterial flora was mainly composed of Bacteroides and Bifidobacterium, which are involved in intestinal fermentation with the production of short-chain fatty acids (SCFA). These short chain fatty acids affected the bone marrow dendritic cell maturation and inhibited the Th2-dependent response, which reduced allergic airway inflammation after allergen exposure.39
The concept of intestinal microbial flora transplantation from healthy donors has also been considered, as this model of treatment has been successfully used in Clostridium difficile-induced inflammation.
It seems that gut microbial biodiversity and diet may exert a preventive effect on allergic diseases, but individual gut and environmental bacteria have also been considered. Early infection by Helicobacter pylori in experimental studies of mice in the neonatal period protected against the development of allergic asthma by the induction of T reg lymphocytes and reprogramming dendritic cells.40Acinetobacter lwoffii F78 and Lactococcus lactis G121, extracted from farm dust, inhibited allergic reactions in mice. These experimental models on animals, which represent complex asthma phenotypes and conditions allow therapeutic or preventive candidates to be better identified.
There are some clinical interventions effective in asthma prevention. In a two-year prospective study of US infants, it has been shown that the mode of delivery influences microbial diversity and composition. Cesarean section significantly altered microbial diversity compared to vaginally born children with significantly lower Bacteroides abundance. Moreover, antibiotic use significantly diminished phylogenetic diversity and richness of bacterial composition of the gut and delayed microbiota maturation. The type of feeding (breastfeeding or formula) also influenced the microbial dysbiosis. These early interventions may increase the risk of allergic diseases.41 The PRACTALL document summarizes the well-documented practical clinical interventions with the impact on human microbiome composition and its preventive role in allergic diseases including avoidance of cesarean section, where possible, reduction in the use of antibiotics in the perinatal and infant period, breastfeeding in order to reduce the risk of dysbiosis, and increase in the consumption of fermentable fibers in diet.32
The idea of primary prevention of asthma and allergy by manipulation of environmental microbial exposures or human microbiome seems a very promising approach; it does, however, also have significant limitations which need to be addressed. One of the most important issues is an early time of potential influence on the immune system. The first months of life or even the prenatal period seem to be the most sensitive for effective intervention. Identification of subjects with a high asthma risk at this early stage would be very important. It is not known how such a preventive effect is stable and if any interventions in later life might be effective.
ConclusionsThere are still many research challenges, such as elucidating the role of fungi and viruses in the prevention of allergic diseases, clarifying the role of microbes in the formation of various phenotypes of allergic diseases and asthma, understanding the interactions between environmental and host microbiome and their influences on immunologic regulations. Therefore, the role of the microbiome in allergy and asthma requires further in-depth research. Simultaneously, this line of inquiry gives hope for the effective prevention and better treatment of these very frequent and chronic diseases.
Funding sourcesNone declared.
Conflict of interestThe authors have no conflict of interest to declare.