Serum IgE evaluation of peanut, hazelnut and walnut allergens through the use of component-resolved diagnosis (CRD) can be more accurate than IgE against whole food to associate with severe or mild reactions.
ObjectivesThe aim of the study was to retrospectively define the level of reaction risk in children with peanut, hazelnut and walnut sensitization through the use of CRD.
Methods34 patients [n=22 males, 65%; median age eight years, interquartile range (IQR) 5.0–11.0 years] with a reported history of reactions to peanut and/or hazelnut and/or walnut had their serum analyzed for specific IgE (s-IgE) by ImmunoCAP® and ISAC® microarray technique.
ResultsIn children with previous reactions to peanut, the positivity of Arah1 and Arah2 s-IgE was associated with a history of anaphylaxis to such food, while the positivity of Arah8 s-IgE were associated with mild reactions. Regarding hazelnut, the presence of positive Cora9 and, particularly, Cora14 s-IgE was associated with a history of anaphylaxis, while positive Cora1.0401 s-IgE were associated with mild reactions. Concerning walnut, the presence of positive Jug r 1, Jug r 2, Jug r 3 s-IgE was associated with a history of anaphylaxis to such food. ImmmunoCAP® proved to be more useful in retrospectively defining the risk of hazelnut anaphylaxis, because of the possibility of measuring Cor a14 s-IgE.
ConclusionsOur data show that the use of CRD in patients with allergy to peanut, hazelnut and walnut could allow for greater accuracy in retrospectively defining the risk of anaphylactic reaction to such foods.
Food allergy is a major public health problem that has familial repercussions on school absences of children and working parents, social interactions and quality of life of the patients. A precise determination of the prevalence of food allergies is very difficult to obtain due to several factors, including methods used in the different studies, geographical, age and dietary exposure. There is a growing awareness of the scientific community about an international increase of this problem in terms of prevalence.1 In Europe, lifetime food allergy estimated prevalence is 17.3% with a maximum prevalence of 6%.2 The persistence of food allergy is variable, the majority of children overcome the allergy to milk and egg; instead, the majority of children allergic to peanut, walnut or shellfish remain allergic throughout their lives. The spectrum of clinical manifestations of food allergy is varied and ranges from mild, local reactions to severe reactions such as anaphylaxis (frequently caused by peanut, hazelnut and walnut allergy), which could be potentially life-threatening.3
Skin prick test (SPT) and serum specific immunoglobulin E (s-IgE) dosage are the first steps of the diagnostic work-up, but s-IgE levels or SPT-wheal size cannot clearly predict whether the patient will have a severe or a mild reaction. They only show sensitization against a specific allergen.4 To date, the gold standard for the diagnosis of food allergies is the double-blind placebo-controlled food challenge (DBPCFC), which is nevertheless time-consuming, expensive and not always safe to perform.1
Laboratory investigations, in combination with the availability of the most common allergenic molecules within the different allergenic sources and advancements in understanding the mechanisms of allergic diseases, have led to molecular diagnostics (i.e. the procedure of quantification of the specific serum IgE against different allergenic molecules present in an allergenic source, including peanut, hazelnut and walnut).
Among allergenic components of peanut, we can recognize some groups of proteins with definite features: pathogenesis-related proteins 10 also called PR-10 (Ara h 8), lipid transfer proteins also known as LTPs (Ara h 9) and storage proteins (Ara h 1, Ara h 2 and Ara h 3). Among allergenic components of hazelnut, we can recognize PR-10 (Cor a 1), LTPs (Cor a 8) and storage proteins (Cor a 9 and Cor a 14). Among allergenic components of walnut, we can recognize LTPs (Jug r 3) and storage proteins (Jug r 1 and Jug r 2).
PR-10 are heat-labile proteins, sensitive to enzymatic digestion and associated with mild reactions, such as oral allergy syndrome (OAS). LTPs and especially storage proteins are heat-stable proteins, resistant to enzymatic digestion and associated with severe reactions, like anaphylaxis.5
The aim of this study was to retrospectively define the level of risk in children with peanut, hazelnut and walnut allergy through the use of CRD.
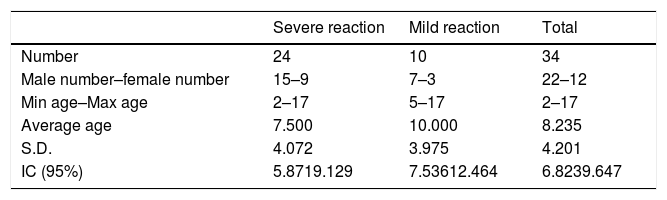
Materials and methodsThe children of the study population were recruited, from July 2013 up to September 2014, among patients who were referred to the Operative Unit of Pediatrics and to the Pediatric Allergology Clinic of the G.B. Rossi University Hospital of Verona. Parents of each pediatric patient signed an informed consent regarding privacy. We enrolled patients who reported a history of symptoms after eating peanuts, hazelnuts or walnuts and who underwent a molecular diagnostic test with ImmunoCAP® (Thermo Fisher Scientific, Uppsala, Sweden) and/or ISAC® (Thermo Fisher Scientific, Uppsala, Sweden) to investigate their allergy as clinically indicated. In our study we recruited 34 patients (minimum age 2, maximum age 17; average age 8.235, S.D. 4.201, IC 95% 6.823–9.647), 22 males (65%, minimum age 2, maximum age 16; average age 8.182, S.D. 3.996, IC 95% 6.512–9.852) and 12 females (35%, minimum age 4, maximum age 17; average age 8.333, S.D. 4.552, IC 95% 5.757–10.909).
We carried out a comparison of the profiles of positivity of s-IgE to the molecular allergens of peanut, hazelnut and walnut, as detected by molecular diagnostics, among a group of patients who had developed severe reactions (anaphylaxis) and another group of patients who had developed mild reactions (OAS), following exposure to peanuts, hazelnuts or walnuts to retrospectively define the level of reaction risk in these children.
We also carried out a comparison between the profiles of the s-IgE positivity to the molecular allergens of hazelnut detected with ISAC® and ImmunoCAP®, in a group of patients who had suffered a severe reaction (anaphylaxis) and another group of patients who had developed mild reactions (OAS), following exposure to hazelnuts to retrospectively compare the usefulness of the two molecular diagnostic techniques in defining the level of reaction risk in these children.
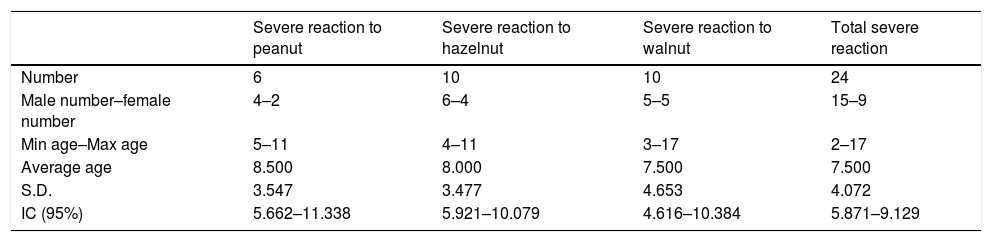
The patients included in the severe reaction study group were 15 males and nine females for a total of 24 patients. The patients included in the mild reaction study group were seven males and three females for a total of 10 patients. (Table 1) Among patients with severe reaction, six patients presented with this kind of reaction to peanut, 10 to hazelnut and 10 to walnut. One patient developed severe reaction to both peanut and hazelnut and another one to both hazelnut and walnut. (Table 2)
Characteristics of severe reaction study group.
| Severe reaction to peanut | Severe reaction to hazelnut | Severe reaction to walnut | Total severe reaction | |
|---|---|---|---|---|
| Number | 6 | 10 | 10 | 24 |
| Male number–female number | 4–2 | 6–4 | 5–5 | 15–9 |
| Min age–Max age | 5–11 | 4–11 | 3–17 | 2–17 |
| Average age | 8.500 | 8.000 | 7.500 | 7.500 |
| S.D. | 3.547 | 3.477 | 4.653 | 4.072 |
| IC (95%) | 5.662–11.338 | 5.921–10.079 | 4.616–10.384 | 5.871–9.129 |
We therefore opted for a qualitative analysis of s-IgE concentrations for different molecular allergens, considering positive molecular tests for s-IgE values >0.35 kUA/L for ImmunoCAP® or >1 ISU-E Units for ISAC®. For computer analysis, we have used Microsoft Excel and Apple Numbers.
ResultsComparison of the profiles of positivity of s-IgE to the molecular allergens of peanut, hazelnut and walnut, as detected by molecular diagnostics, among the group of patients who had developed severe reactions (anaphylaxis) and the group of patients who had developed mild reactions (OAS), following exposure to peanuts, hazelnuts or walnuts to retrospectively define the level of reaction risk in these children are reported in Tables 3 and 4.
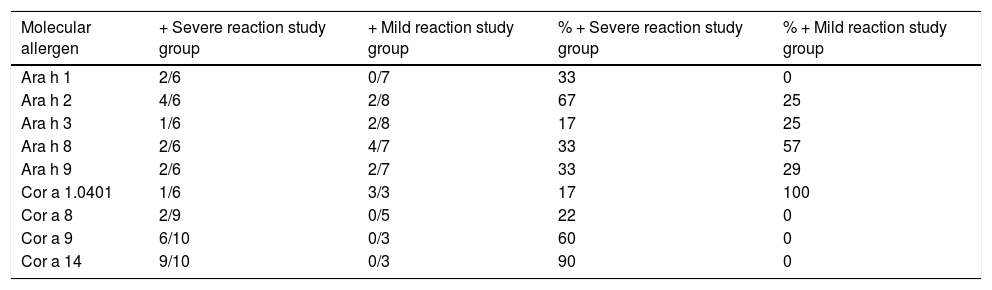
Analysis of s-IgE positivity to peanut and hazelnut molecular allergens through ImmunoCAP®.
| Molecular allergen | + Severe reaction study group | + Mild reaction study group | % + Severe reaction study group | % + Mild reaction study group |
|---|---|---|---|---|
| Ara h 1 | 2/6 | 0/7 | 33 | 0 |
| Ara h 2 | 4/6 | 2/8 | 67 | 25 |
| Ara h 3 | 1/6 | 2/8 | 17 | 25 |
| Ara h 8 | 2/6 | 4/7 | 33 | 57 |
| Ara h 9 | 2/6 | 2/7 | 33 | 29 |
| Cor a 1.0401 | 1/6 | 3/3 | 17 | 100 |
| Cor a 8 | 2/9 | 0/5 | 22 | 0 |
| Cor a 9 | 6/10 | 0/3 | 60 | 0 |
| Cor a 14 | 9/10 | 0/3 | 90 | 0 |
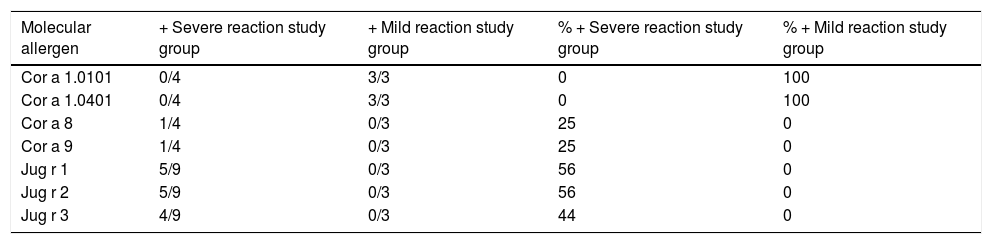
Analysis of s-IgE positivity to hazelnut and walnut molecular allergens molecular allergens through ISAC®.
| Molecular allergen | + Severe reaction study group | + Mild reaction study group | % + Severe reaction study group | % + Mild reaction study group |
|---|---|---|---|---|
| Cor a 1.0101 | 0/4 | 3/3 | 0 | 100 |
| Cor a 1.0401 | 0/4 | 3/3 | 0 | 100 |
| Cor a 8 | 1/4 | 0/3 | 25 | 0 |
| Cor a 9 | 1/4 | 0/3 | 25 | 0 |
| Jug r 1 | 5/9 | 0/3 | 56 | 0 |
| Jug r 2 | 5/9 | 0/3 | 56 | 0 |
| Jug r 3 | 4/9 | 0/3 | 44 | 0 |
From the analysis of s-IgE levels against peanut molecular allergens through ImmunoCAP®, positivity emerged to Ara h 1 in two out of six (33%), to Ara h 2 in four out of six (67%) and to Ara h 3 in one out of six (17%) patients with a severe reaction. Five out of six (83%) patients of the severe reaction study group were associated with positivity to Ara h 1, 2 and 3. ImmunoCAP® test also revealed positivity to the PR-10 protein family and Bet v 1-homologous Ara h 8 in four out of seven patients (57%) with mild reaction, a closeness of the percentages of positivity to Ara h 9 obtained by the two study groups (two out of six patients, 33%, for the severe reaction study group, and two out of seven patients, 29%, for the mild reaction study group), but higher positivity rate for Ara h 3 in the mild reaction study group (two out of eight patients, 25%) compared to the severe reaction study group (one out of six patients, 17%). (Table 3)
From the analysis of s-IgE levels against hazelnut molecular allergens through ISAC®, positivity emerged to the LTP Cor a 8 in one out of four patients (25%) and to the storage protein Cor a 9 in one out of four patients (25%) with a severe reaction. Only two out of four (50%) patients of the severe reaction study group were associated with positivity to Cor a 8 and 9. ISAC® also highlighted positivity to the PR-10 protein family and Bet v 1-homologous Cor a 1.0401 in three out of three patients (100%) and Cor a 1.0101 in three out of three patients (100%) with mild reaction. (Table 4)
From the analysis of hazelnut molecular allergens through ImmunoCAP®, positivity emerged to the LTP Cor a 8 in two out of nine patients (22%), to the storage of protein Cor a 9 in six out of 10 patients (60%) and to the storage protein Cor a 14 in nine of 10 patients (90%) with a severe reaction. All patients (10) in the severe reaction study group were associated with positivity to Cor a 8, 9 and 14. ImmunoCAP® also highlighted positivity to the PR-10 protein family and Bet v 1-homologous Cor a 1.0401 in three out of three patients (100%) with a mild reaction and proximity of the percentages of positivity for Cor a 8 in the mild reaction study group (two out of nine patients, 22%) compared to the severe reaction study group (zero out of five patients, 0%). (Table 3)
From the analysis of s-IgE levels against walnut molecular allergens through ISAC®, positivity emerged to the storage protein Jug r 1 in five out of nine patients (56%), to the storage of protein Jug r 2 in five out of nine patients (56%) and to the LTP Jug r 3 in four out of nine patients (44%) with a severe reaction. All the patients (9) of the severe reaction study group were associated with positivity to Jug r 1, 2 and 3. (Table 4)
DiscussionPeanutResults from the analysis of s-IgE levels against peanut molecular allergens Ara h 1, 2 and 3 through ImmunoCAP® are in agreement with those found in literature, according to which the sensitization to such storage proteins is associated with severe systemic immediate reactions.6–10 In our study, Ara h 2 proved to be the component with greatest discrimination capability between severe and mild reactions to peanut, as already reported by several studies in literature.11–14
Results from the analysis of s-IgE levels against peanut molecular allergen Ara h 8 through ImmunoCAP® are in agreement with what has been reported in literature, where the sensitization to that molecular allergen is considered a marker of primary sensitization to pollen and, from a clinical point of view, of tolerance in moderate or local reactions to peanut.15–18
The closeness of the percentages of positivity to Ara h 9 obtained by the two study groups is also in agreement with the literature. In fact, Ara h 9 belongs to the LTP family and is considered a marker of severe systemic reactions as well as of oral allergy syndrome.18
Our study also showed a higher positivity rate for Ara h 3 in the mild reaction study group compared to the severe reaction study group, but this is in disagreement with what has been reported in literature. This discrepancy could be explained by the low number of patients included in the study groups.
HazelnutResults from the analysis of s-IgE levels against hazelnut molecular allergens Cor a 8 and 9 through ISAC® are not in agreement with those found in the literature according to which the sensitization against these molecular allergens has been associated with severe immediate signs and symptoms.19–21 This inadequate result could be explained by the low number of patients included in the study groups, in addition to the absence of Cor a 14 among the molecular allergens included in the panel tested with ISAC®.
Results from the analysis of s-IgE levels against hazelnut molecular allergens Cor a 1.0401 and 1.0101 through ISAC® are in agreement with what has been reported in literature, where the sensitization to that molecular allergy is again considered a marker of primary sensitization to pollen and, from a clinical point of view, of tolerance in moderate or local reactions to hazelnut, such as oral allergy syndrome.19,20
Results from the analysis of s-IgE levels against hazelnut molecular allergens Cor a 8, 9 and 14 through ImmunoCAP® are in agreement with those found in literature according to which the sensitization to these molecular allergens has been associated with severe immediate signs and symptoms.19,20 In our study, Cor a 14 proved to be the component with greatest discrimination capability between mild and severe reaction to hazelnut.22,23
Results from the analysis of s-IgE levels against hazelnut molecular allergen Cor a 1.0401 through ImmunoCAP® are in agreement with what has been reported in literature, where the sensitization to that molecular allergen is again considered a marker of primary sensitization to pollen and, from a clinical point of view, of tolerance in moderate or local reactions to hazelnut, such as oral allergy syndrome.19,20
The proximity of the percentages of positivity for Cor a 8 in the mild reaction study group compared to the severe reaction study group could again be explained by the low number of patients included in the study groups.
Then, we also carried out a comparison between the profiles of the s-IgE positivity to the molecular allergens of hazelnut detected by ISAC® and ImmunoCAP®, in a group of patients who had suffered a severe reaction and another group of patients who had developed mild reactions, following exposure to hazelnuts to retrospectively compare the usefulness of the two techniques in defining the level of reaction risk in these children, noting discrepancies in the results obtained between them.
From the analysis of s-IgE levels to hazelnut molecular allergens through ISAC®, positivity emerged to the LTP Cor a 8 in one out of four patients (25%) and to the storage protein Cor a 9 in one out of four patients (25%). Alternatively, using ImmunoCAP®, positivity emerged to the LTP Cor a 8 in two out of nine patients (22%), to the storage protein Cor a 9 in six out of 10 patients (60%) and to the storage protein Cor a 14 in nine out of 10 patients (90%) with a severe reaction. Only two out of four (50%) patients of the severe reaction study group were associated to positivity to Cor a 8 and 9 through ISAC®. Alternatively, all the patients (10) of the severe reaction study group were associated with positivity to Cor a 8, 9 and 14 through ImmunoCAP®.
This essential difference between the two molecular diagnostic techniques is due to the efficiency of detection of s-IgE to Cor a 14 through ImmunoCAP®, which is absent in the panel of molecular allergens potentially testable with ISAC®. In fact, the determination of s-IgE to Cor a 8 and 9 through ISAC® and ImmunoCAP® does not justify this discrepancy.
From the analysis of s-IgE levels against hazelnut molecular allergens through ISAC®, positivity emerged to the PR-10 protein family and Bet v 1-homologous Cor a 1.0401 in three out of three patients (100%) and Cor a 1.0101 in three out of three patients (100%). Alternatively, through ImmunoCAP®, positivity emerged to the PR-10 protein family and Bet v 1-homologous Cor a 1.0401 in three out of three patients (100%) with a mild reaction.
WalnutResults from the analysis of s-IgE levels against walnut molecular allergens Jug r 1, 2 and 3 through ImmunoCAP® are in agreement with those found in literature, according to which the sensitization to such proteins is associated with serious reactions.24
ConclusionsThe use of CRD in patients with allergy to peanut, hazelnut and walnut could allow for greater accuracy in retrospectively defining the risk of an anaphylactic reaction to such foods.
For children with reactions to peanut, the presence of positive s-IgE against Ara h 1 and Ara h 2 is associated with a history of anaphylaxis to such food, while positive s-IgE against Ara h 8 is associated with OAS. Regarding hazelnut, the presence of positive s-IgE against Cor a 9 and, especially, against Cor a 14 is associated with a history of anaphylaxis to such food, while positive s-IgE against Cor a 1.0401 is associated with OAS. Between the two CRD techniques, ImmmunoCAP® proved to be more useful in retrospectively defining patients at high risk of hazelnut anaphylaxis, because of the possibility to dose s-IgE against Cor a 14, which is not present in the ISAC® panel. Consequently, this study could suggest to the clinician to choose ImmunoCAP® to assess the level of risk in a patient allergic to hazelnuts, or, on the other hand, that the ISAC® panel could be improved with the inclusion of Cor a 14 in its panel of molecular allergens. Concerning walnut, the presence of positive s-IgE against Jug r 1, Jug r 2 and Jug r 3 is associated with a history of anaphylaxis to such food.
Our study suffered from the low number of patients included in the study groups, a factor that has not allowed us to have statistically significant quantitative results for the concentrations of s-IgE against the different molecular allergens. This limit can be explained by the restricted use of the CRD techniques (only in selected cases), as indicated in clinical practice, and because of their considerable economic weight in times of spending review. However, our analysis proves to be substantially in line with what has been reported in literature.
Contributors’ statementDr. Giovannini acquired the data, analyzed and interpreted the data, drafted the article and approved the final version of the manuscript as submitted.
Dr. Comberiati analyzed and interpreted the data, revised the article critically for important intellectual content and approved the final version of the manuscript as submitted.
Dr. Piazza analyzed and interpreted the data, revised the article critically for important intellectual content and approved the final version of the manuscript as submitted.
Dr. Chiesa acquired the data, revised the article critically for important intellectual content and approved the final version of the manuscript as submitted.
Prof. Piacentini analyzed and interpreted the data, revised the article critically for important intellectual content and approved the final version of the manuscript as submitted.
Prof. Boner analyzed and interpreted the data, revised the article critically for important intellectual content and approved the final version of the manuscript as submitted.
Dr. Zanoni conceptualized and designed the study, analyzed and interpreted the data, revised the article critically for important intellectual content and approved the final version of the manuscript as submitted.
Prof. Peroni conceptualized and designed the study, analyzed and interpreted the data, revised the article critically for important intellectual content and approved the final version of the manuscript as submitted.
Ethical disclosuresConfidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentAll the patients and/or subjects included in the study have received sufficient information and have given their informed consent to participate in that study.
Protection of human subjects and animals in researchThe authors declare that no experiments were performed on humans or animals for this investigation.
Financial disclosureAll authors have no financial relationships relevant to this article to disclose.
Founding sourceThis research received no specific grant from ant funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interestNone declared.
This work was performed at Department of Surgical, Stomatologic and Mother-Child Sciences, Section of Pediatrics, University of Verona, Verona, Italy.