Component resolved diagnosis (CRD) allows to precisely identify the sensitization to specific molecules of a given allergenic source, resulting in an important improvement in clinical management, particularly of polysensitized subjects. This will end in the correct prescription of allergen immunotherapy (AIT) for respiratory allergy and in adequate avoidance diets or prescription of self-injectable adrenaline in food allergy.
ObjectiveThe aim of this multicenter, real life study is to evaluate the percentage change of the diagnostic-therapeutic choice in polysensitized patients with respiratory allergy and in patients with food allergy, after using CRD compared to a first level diagnosis, along with an economic analysis of the patient's overall management according to the two different approaches.
MethodsAn overall number of 462 polysensitized patients, as suggested by skin prick tests (SPT), and with clinical symptoms related to a respiratory (275 pts) or food (187 pts) allergy, were recruited. All patients underwent CRD for specific IgE against food or inhalant recombinant molecules, which were chosen according to medical history and positivity to SPT. The first diagnostic-therapeutic hypothesis, based only on medical history and SPT, was recorded for each patient while the final diagnostic-therapeutic choice was based on the results from CRD. The rate of change of the diagnostic-therapeutic choice from the first hypothesis to the final choice was statistically evaluated. The economic impact of CRD on the overall management of the allergic patients was analyzed to evaluate whether the increase in the diagnostic costs would be compensated and eventually exceeded by savings coming from the improved diagnostic-therapeutic appropriateness.
ResultsAn approximate 50% change (k index 0.54) in the prescription of AIT for respiratory allergy as well as a change in the prescription of self-injectable adrenaline (k index 0.56) was measured; an overall saving of financial resources along with a higher diagnostic-therapeutic appropriateness was also detected.
ConclusionThere is moderate agreement concerning prescription of AIT and self-injectable adrenaline before and after performing CRD: this highlights the usefulness of CRD, at least in polysensitized patients, in indicating the risk assessment and therefore the correct therapy of respiratory and food allergy, which results in a cost-saving approach.
Respiratory allergy, which includes allergic rhinitis (AR) and bronchial asthma, and food allergy, are the most common hypersensitivity diseases. Epidemiological studies showed that the increase in the prevalence of asthma and AR observed in several Western nations since the 1960s–1970s,1,2 was accompanied in the latest decades by a similar increase in food allergy,3 reaching the point of defining it as the “second wave” of the allergic epidemic. On a worldwide level, based on several studies, food allergy seems to concern about 5–8% of children and 2–5% of adults, and from a minimum of 1–2% to a maximum of 10% of the general population.4–7 As far as anaphylaxis is concerned, a number of studies conducted in recent years especially in the USA, confirmed a relevant increase of its incidence, obtained from related admissions to hospitals, both in young people8 and in children,9,10 with foods as the main trigger cause. These pathologies, despite not usually being associated to serious morbidity and mortality, generate a burden of illness particularly meaningful on society, because of the impact on the quality of life (QoL) and because of the cost of treatment, therefore representing a public health issue,11,12 with obvious repercussions on sanitary systems and on the population itself, both in social terms and strictly economic terms as well (increase of direct, indirect, intangible and opportunity costs).11–24 The need to achieve a diagnosis as precise as possible in identifying the causative allergen proves of fundamental importance, in order to set up the most adequate therapy. Traditional diagnostic tests are aimed at demonstrating the presence of specific IgE against one or more allergenic extracts, through two modalities: in vivo, by performing skin prick tests (SPT), or in vitro, by the detection of specific IgE (sIgE) in blood by ImmunoCAP methodology.11 In particular, SPT represents the first level diagnostic tool, mainly because of its minimally invasive procedure and modest costs.25 However, both cutaneous and in vitro tests yield a fair sensitivity (70–100%) but an unsatisfactory specificity (40–70%),26 and can therefore result in a high number of false positive results. As a consequence, in food allergy an oral food challenge is often required, which is a third level test allowing a sound diagnosis, but not being within the reach of all allergy centers. Furthermore, in recent years, it has been understood how the allergenic extracts used until now are actually composed by a mixture of allergenic molecules, some common to multiple allergenic sources (named cross-reactive molecules or panallergens) while other are specific of a singular source (named genuine molecules or species-specific allergens).27 Therefore, the use of new methods offered by the so-called Component Resolved Diagnosis (CRD), that allow the detection of sIgE against single recombinant molecules and to identify the IgE sensitization also to the single molecules, gives a profile of individual reactivity to IgE for a sensitized patient. As well as being underlined by the Consensus document on molecular-based allergy diagnostics of the World Allergy Organization,28 published in 2013, the use of CRD is aimed at achieving three main objectives: (1) distinguishing genuine versus cross-reactive sensitization in polysensitized patients, thereby improving the knowledge of the real triggering allergens; (2) assessing the risk of severe, systemic versus mild local reactions in food allergy, thus reducing unnecessary anxiety for the patient and the need to perform oral food challenges; (3) identifying the allergens responsible for symptoms and therefore the suitable patient for allergen immunotherapy (AIT). Each of these objectives has remarkable implications, both on diagnosis and prognosis, in respiratory allergy as well as in food allergy. In respiratory allergy, the possibility of defining with molecular precision the triggering allergen implicates on one side a diagnosis of greater accuracy of the causal role of the allergen itself and on the other side, since the products for AIT are standardized on genuine allergens (e.g., Phl p 1-5 of grass family or Bet v 1 of Betulaceae), a more correct indication for AIT, which through its disease-modifying effect has also a recognized cost-saving profile compared to drug therapy.29–31 This is even more important in patients who are seemingly polysensitized, this making clinical interpretation more difficult, based on the capacity of CRD to differentiate the genuine sensitizations, affecting symptomatology and hence deserving AIT, from those due to cross-reactivity, which are not particularly relevant from a clinical point of view but may result in the prescription of inappropriate AIT.32–34 Also in food allergy the possibility of defining with molecular precision the triggering allergen implicates a diagnosis of greater certainty of the causal role of the allergen itself in triggering the symptoms; this permits to identify patients with true allergy (clinically relevant) to that food. Moreover, in this field, such diagnostics allow to investigate the IgE-mediated reactivity to food allergenic sources potentially cross-reactive but, in particular, it enables to classify the symptomatic patient, monosensitized or polysensitized, into risk classes of different clinical severity depending on the type of sensitizing molecule (e.g., degree of heat stability and of resistance to proteolytic digestion). This will have as a consequence, in case of high risk, the need to prescribe an avoidance diet and possibly self-injectable adrenaline, while, in the case of low risk, the opportunity to reconsider the real necessity of following a given diet; in both cases, it will be possible to avoid the performance of oral food challenges and the consequences are relevant for patients both in the socio-economic and in the clinical aspect.35–37 Our study was aimed at evaluating the percentage change of the diagnostic-therapeutic choice in polysensitized patients affected by food or respiratory allergy, after using CRD compared to a first level survey and also at carrying out an economic analysis of the patient's overall management according to the molecular approach, with detailed analysis of higher costs and savings in respect to traditional methodologies, from the perspective of an optimization of the resources. To our knowledge, no study assessing the role of CRD in the dual aspect of improving diagnostic-therapeutic appropriateness and optimizing financial resources, both for respiratory and food allergy, is available. In fact, taken singularly, recent papers addressed the clinical role of CRD in respiratory allergy,38–40 while others focused on an economical evaluation.41,42 Only one study43 has dealt with food allergy but did not include an economic evaluation.
MethodsSelection of patientsThe selection of patients for the clinical diagnostic prospective cohort study in a real-life setting was carried out in three allergy centers (Piacenza, Pordenone, Mantova) from August 18, 2015 to October 10, 2015. As potential candidates, all patients of the respective outpatient clinics who underwent SPT during a first allergy visit were considered. The inclusion in the study required the coexistence of the following criteria: (1) polysensitization to SPT, indicating the presence of two or more sensitizations to food or aeroallergens respectively; (2) the presence of clinical symptoms related to respiratory allergy (rhinoconjunctivitis and/or bronchial asthma) or food allergy (oral allergy syndrome and/or urticaria-angioedema and/or abdominal pains-nausea-vomit-diarrhea and/or rhinoconjunctivitis-bronchospasm and/or cardiovascular symptoms). Globally, 462 patients were recruited (aged from 10 months to 83 years), of whom 275 were suffering from respiratory allergy and 187 from food allergy. All the investigations were performed under the regular routine evaluation of polysensitized allergic patients; no drug was used in the study. This study was notified to the local ethics committees, according to the national law.
Methodology of the studyEach patient underwent the following examinations:
- (1)
SPT for inhalants (dust mites, Alternaria, Aspergillus, Cladosporium, cat, dog, grass, birch, ragweed, mugwort, Parietaria, plantain, olive, and cypress pollens, latex) or foods (milk, egg, wheat, cod, shrimp, peach, apple, tomato, peanut, soy, hazelnut, nut) (Alk®, Hørsholm, Denmark; Stallergenes Greer®, London, England); the wheals with diameter ≥3mm were considered positive, in absence of positivity to negative control (glycerol-saline solution); positive control was histamine (10mg/ml);
- (2)
Blood sampling to detect sIgE against food or inhalant recombinant molecules (CRD), which were chosen according to medical history and positivity to SPT (available molecules were Phl p 1-5-7-12, Art v 1, Amb a 1, Par j 2, Bet v 1, Ole e 1, Der p 1-2, Alt a 1, Pru p 3, Cor a 8-9-14, Jug r 1-3, Ara h 1-2-3-9, Gly m 5-6, Mal d 3, Tri a 14-19, Gal d 1-2, Casein, Pen a 1, Cyp c 1, Hev b 1-3-5-6.01, Can f 1, and Fel d 1), by ImmunoCAP method (Thermo Fisher Scientific®, Uppsala, Sweden), a fluorescence enzyme test (FEIA) built on the technique of “sandwich” immunoassay (quantitative result in kUA/L, positive if ≥0.10kUA/L). Only in cases when chosen molecules were not available with the ImmunoCAP methodology (e.g., Cup a 1, Pla l 1), the serum was instead analyzed with the Microarray ISAC 112 allergens system (Thermo Fisher Scientific®, Uppsala, Sweden), based on a high number of allergenic molecules arranged on microarray on a solid glass support (four reaction sites involved), with use of human anti-IgE antibodies labeled with a fluorochrome and measure of the fluorescent part from a laser scanner (semi-quantitative result in ISU/L, positive if ≥0.03 ISU/L).
For each patient the following was recorded: (1) the first diagnostic-therapeutic hypothesis, i.e. the one that would be carried out based solely on the first level testing (medical history and SPT) possibly identifying the triggering allergen(s) driving the choice of allergens to be used for AIT for respiratory allergy, and the potential prescription of an avoidance diet, as well as the potential prescription of self-injectable adrenaline for food allergy; (2) the second diagnostic-therapeutic choice (final), i.e. the one that would be implemented considering the CRD outcome. Therefore, an evaluation of the percentage of change of the diagnostic-therapeutic choice was carried out, comparing the first hypothesis and the final choice, and dividing the patients into three categories: (1) patients for whom the molecular approach did not cause changes of any kind concerning the diagnostic-therapeutic decision; (2) patients for whom the outcome of CRD refined the diagnosis, that is, a better definition of the triggering allergen(s)] but did not substantially influence the therapy; (3) patients for whom the CRD induced to modify not only the diagnosis but also the therapeutic choice (prescription of AIT or avoidance diet/adrenaline).
Statistical analysisA descriptive statistical investigation was done. Furthermore, a statistical analysis was performed by calculation of agreement coefficient (k index) on obtained results in terms of patients who underwent AIT, before and after molecular diagnostics, as well as on the results obtained in terms of adrenaline devices prescribed before and after CRD. The data were interpreted according to the Landis & Koch scale, which states that the finding of a low agreement supports the usefulness of the molecular survey in the precision of the allergy diagnostics, in particular, k=0.81–1.00, almost perfect; 0.61–0.80, substantial; 0.41–0.60, moderate; 0.21–0.40, fair; 0.00–0.20, slight; and ≤0.001, poor. The chi-square test was used to evaluate the difference in the number of modified diagnosis and therapies according to sensitization toward perennial or seasonal inhalant allergens. A type one error of 5% was accepted.
Economical evaluationA detailed analysis was conducted on the economic impact of a molecular approach to the overall management of the allergic patients in order to evaluate whether the increase in the diagnostic costs would be compensated and eventually exceeded by savings coming from the increasing diagnostic-therapeutic appropriateness. At this regard, the average cost of CRD execution, about €50 for each patient with respiratory allergy (5.5 molecules for €9 per molecule),41 and the cost of a minimum three-year course of AIT, estimated to be about €100041 were taken into account. The costs related to allergy visits and SPTs were not considered, since they are common to both diagnostic approaches, as well as the costs related to the symptomatic drug therapy, to potential absences from work and, generally speaking, to the changes of the quality of life (QoL), although they are considered relevant from literature.44
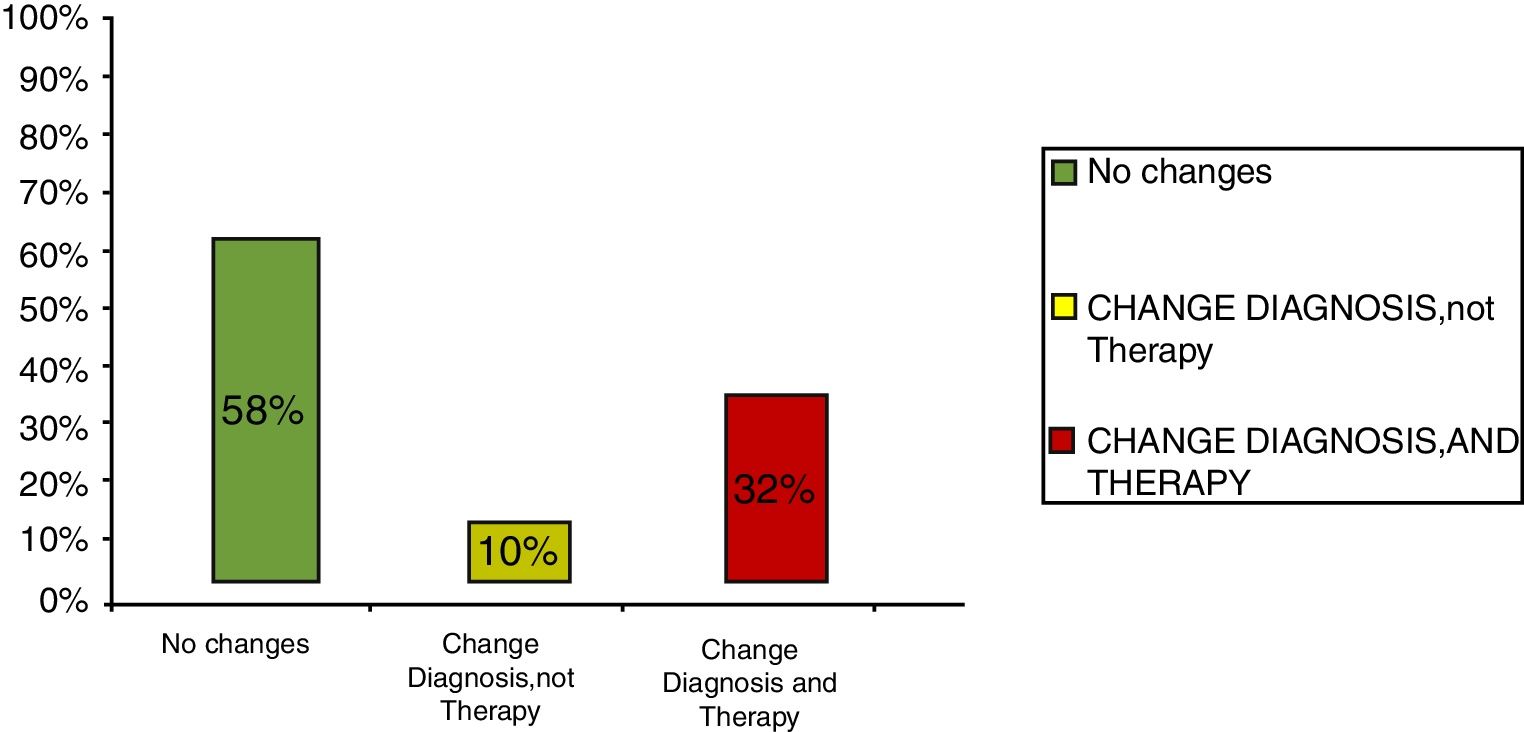
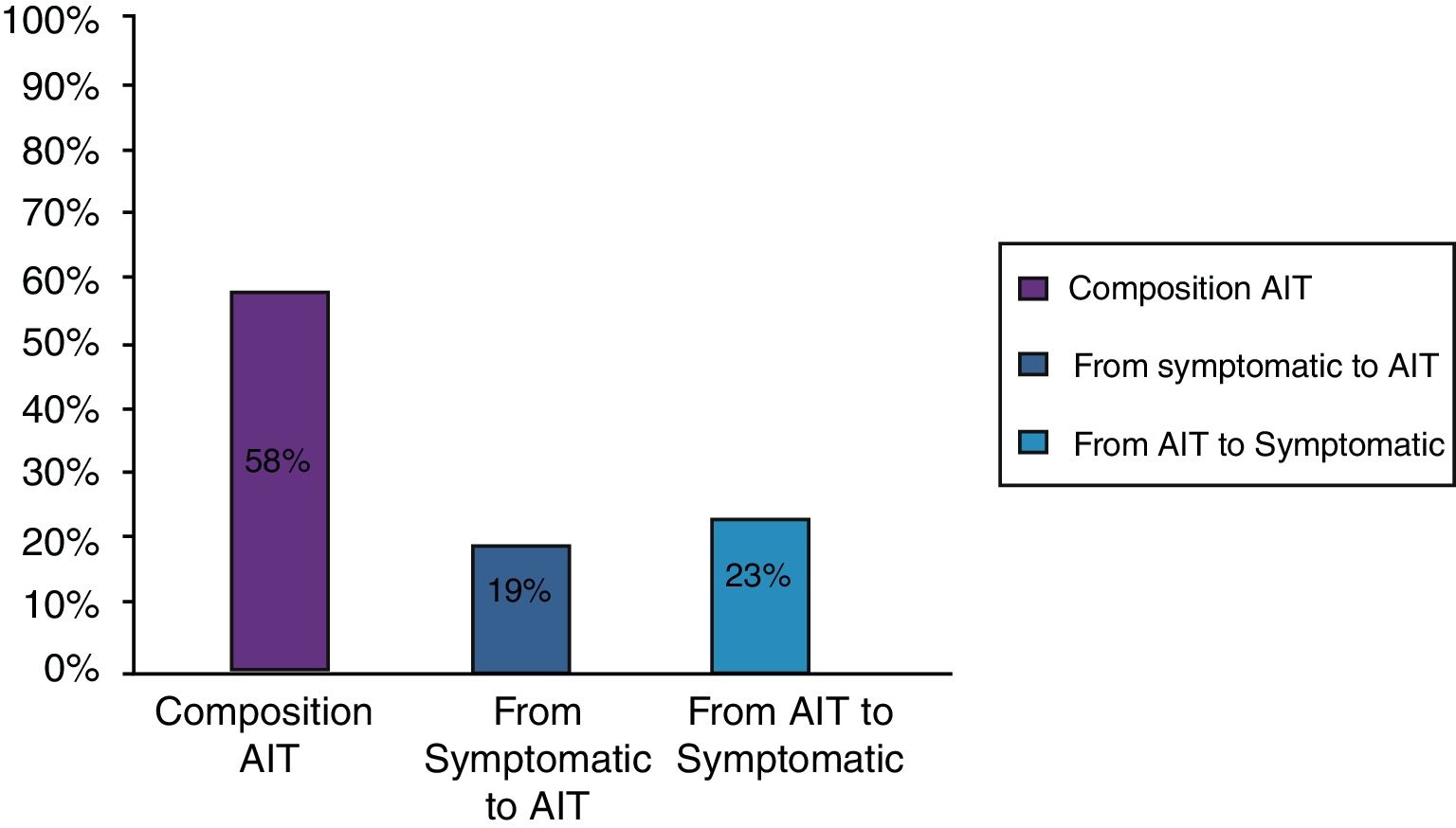
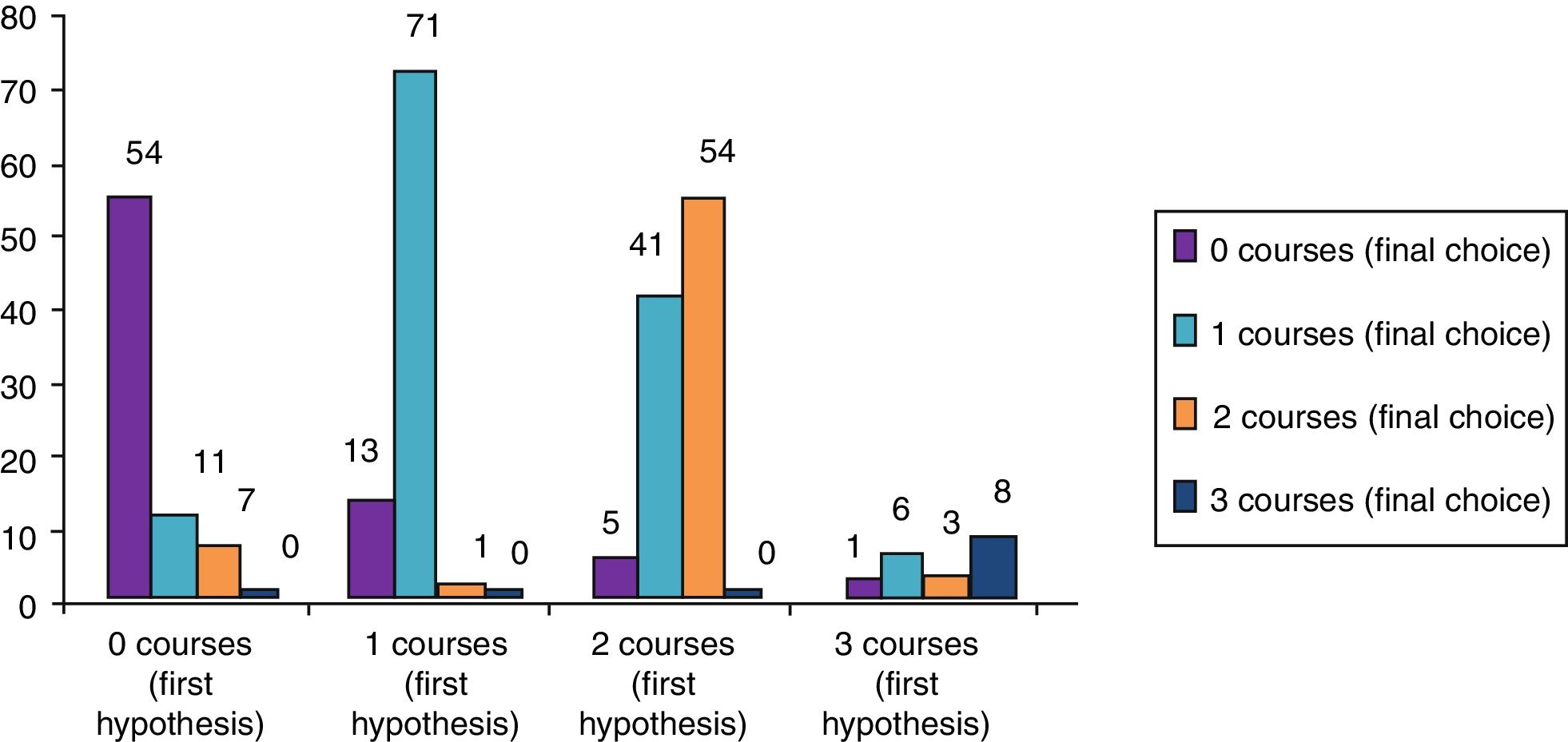
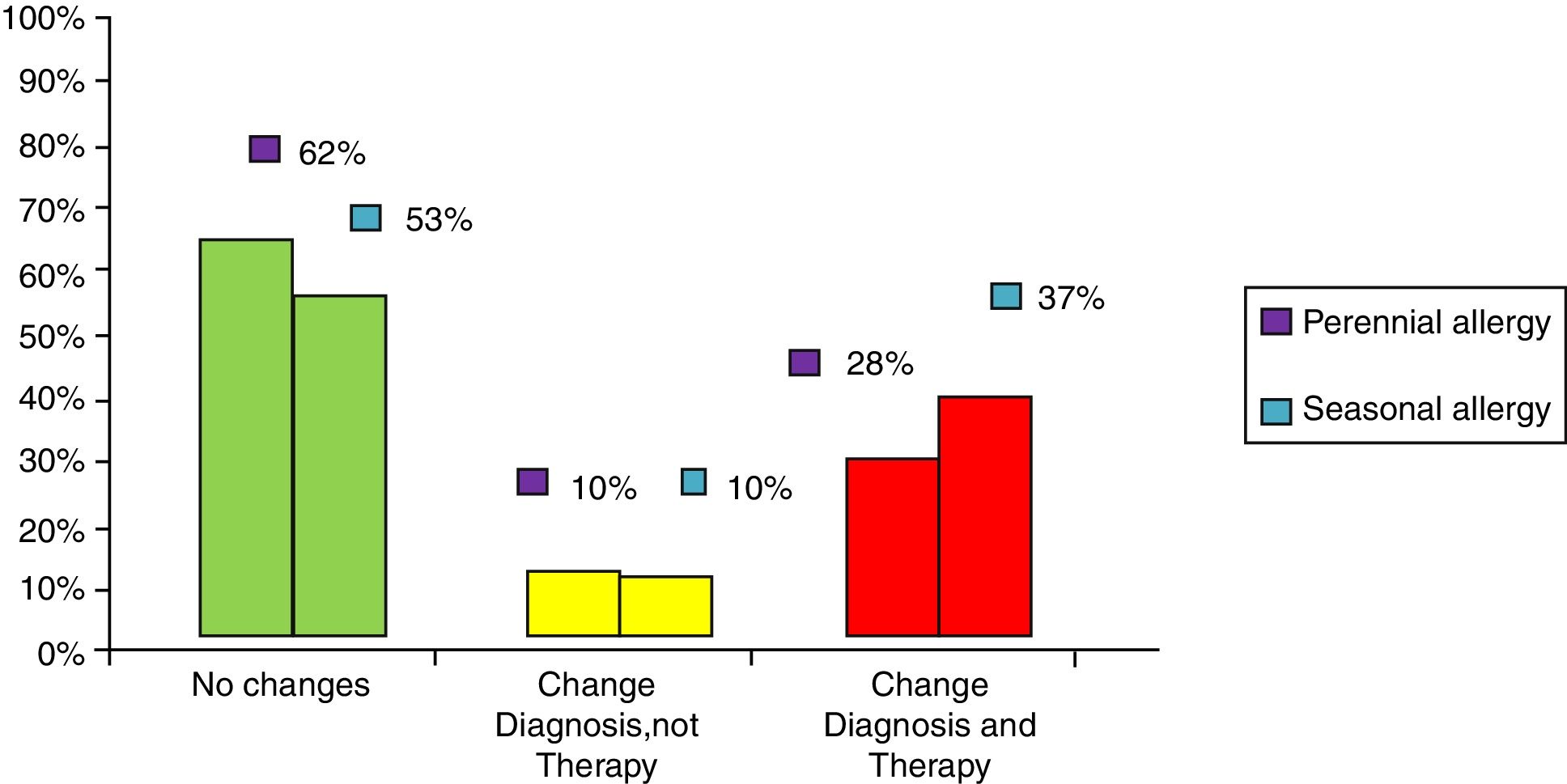
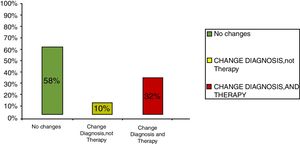
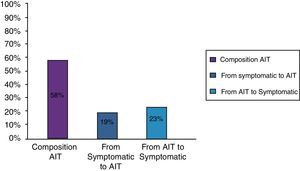
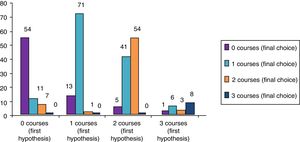
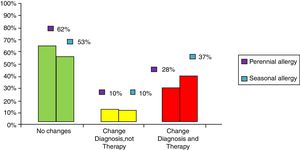
ResultsClinical results in respiratory allergyRegarding the 275 patients affected by respiratory allergy, the implementation of CRD did not cause changes to the diagnostic-therapeutic choice in 160 of them (58%), while it resulted in refining the diagnosis (D) or an overall change of diagnosis and therapy (D and T), respectively in 27 (10%) and in 88 (32%) patients (Fig. 1). Statistically, the analysis of the correlation between the two diagnostic methods (traditional diagnostics vs. molecular approach) in identifying patients who are prescribed AIT highlights a concordance between the methods (the proportion of patients for whom the two methods agree in the formulation of clinical judgment on the total of patients) amounting to 68% of cases; the agreement coefficient (k index) amounts to 0.54, meaning a moderate agreement. Furthermore, taking only the 88 patients who experienced a change of therapeutic decision into consideration, in 51 of them (58%) the AIT prescription changed from trees to grasses and from trees to cypress based on positive response for genuine molecules, while in 42% AIT became the chosen treatment (instead of drugs) in 17 patients (19%), while in 20 patients (23%) the opposite occurred, i.e. drugs were prescribed instead of AIT (Fig. 2). Indeed, as many as 41 of 100 patients that would undergo two AIT courses with the traditional diagnostic method, eventually underwent only one course (Fig. 3). On the contrary, most patients who underwent no course or one course of AIT, respectively 54 of 72 and 71 of 85, kept going through the same number of courses. Instead, separately evaluating the patients with perennial allergy, which were those who were sensitized to dust mites and/or molds (149), compared to those with seasonal clinical symptoms (126), it is deduced that in 93 patients (62% of the perennial) vs. 67 (53% of seasonal) there was no change in the diagnostic-therapeutic choice, in 15 (10%) vs. 12 (10%) the diagnosis (D) was refined, while in 41 (28%) vs. 47 (37%) there was a modification of diagnosis and therapy (D and T) (Fig. 4). Statistically, the chi-square test for the difference between seasonal and perennial was not significant (p=0.12). Lastly, when we analyzed the profile of molecular sensitization of the 275 patients with respiratory allergy, 84 of them (31%) resulted sensitized to at least one panallergen (profilin or polcalcin); in 38 of these 84 patients there was an overall change of diagnosis and therapy (D and T). CCD reactivity was evaluated in all patients and four sensitizations (0.01%) were found only in subjects with positivity to recombinant but not native molecules.
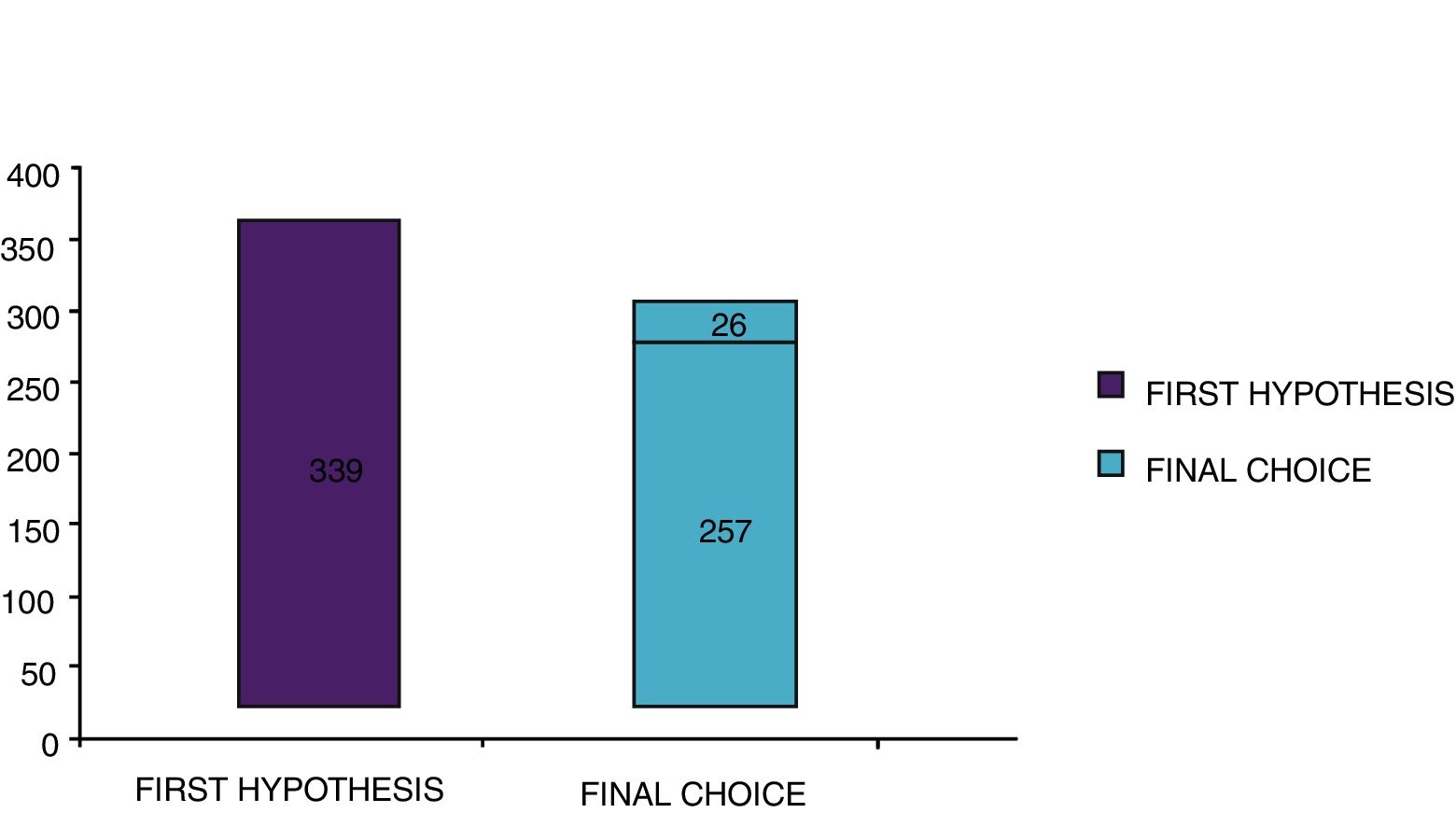
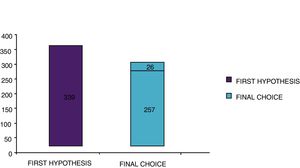
With the traditional diagnostic method (first diagnostic-therapeutic hypothesis) a total of 339 minimum three-year courses of AIT were prescribed, while according to the molecular approach (final choice) the therapeutic courses were reduced to 283 (comprehensive of 26 courses prescribed only after CRD), therefore with a decrease of 56 units (Fig. 5). Taking into account that a three-year AIT course costs about 1000 euros,41 the immunotherapy expense changed from €339,000 to €283,000, with a saving of €56,000. Then, deducting the higher costs of the diagnostic phase of molecular approach (€50 euros for each of 275 patients, total amounting to €13,750),41 the final saving in respiratory allergy was estimated to be €42,250.
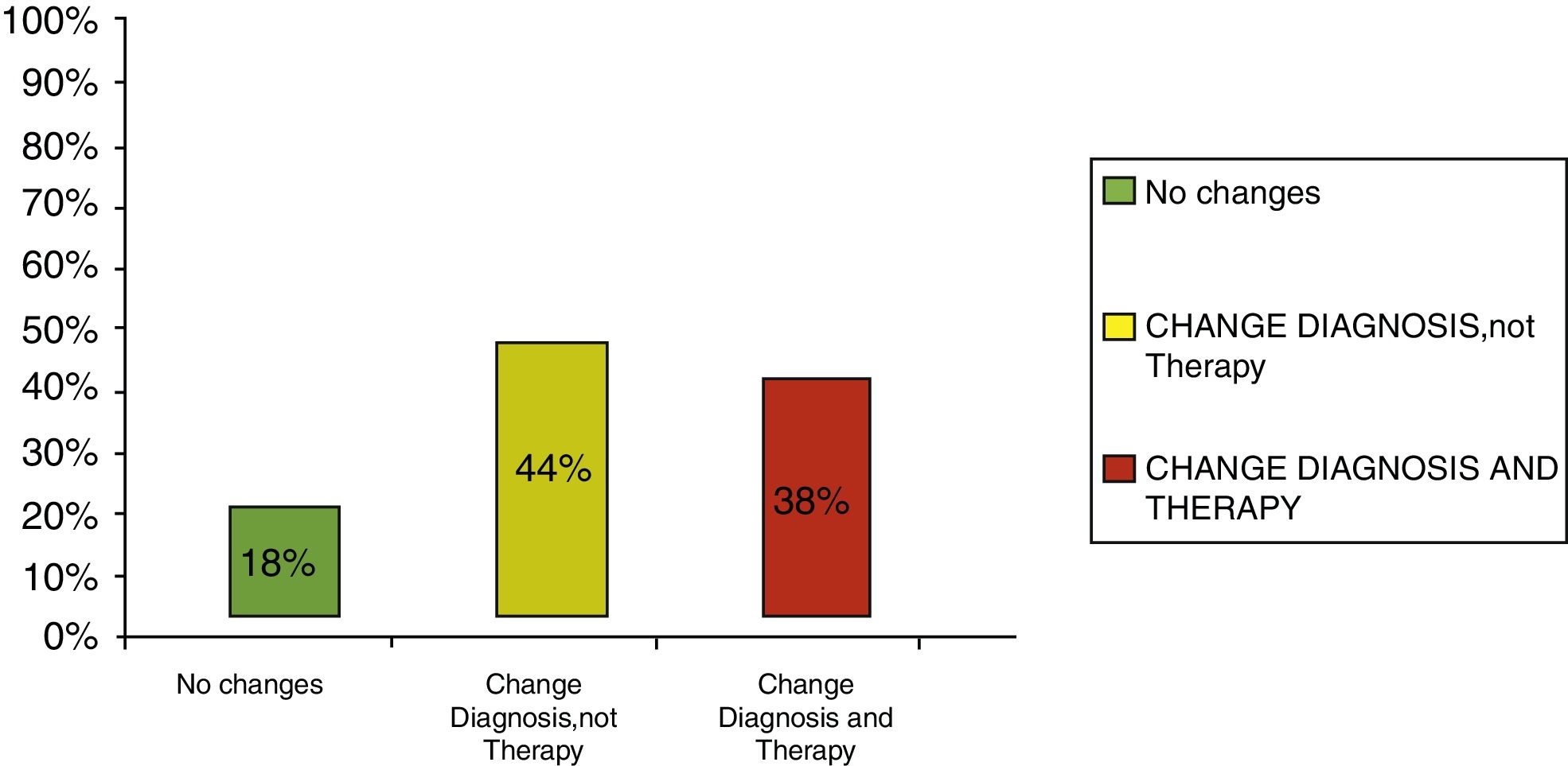
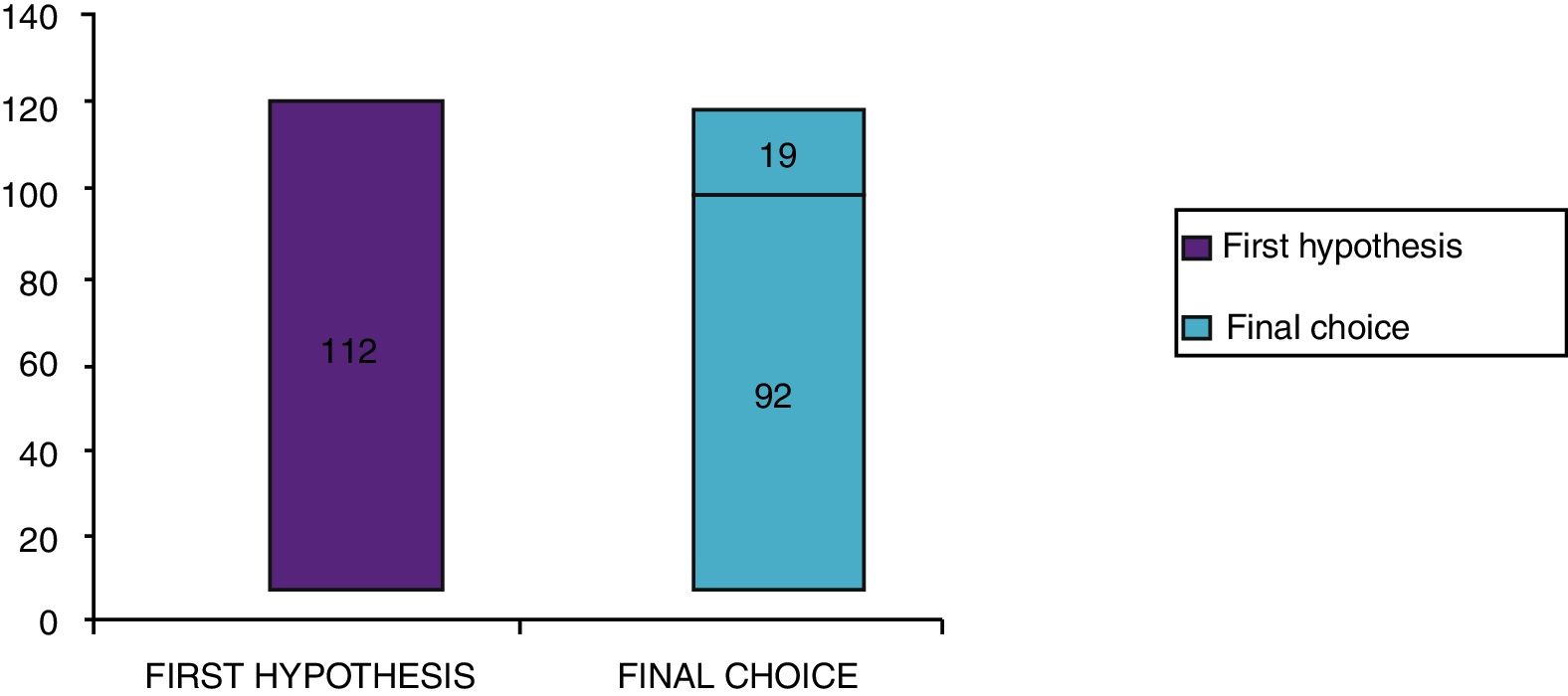
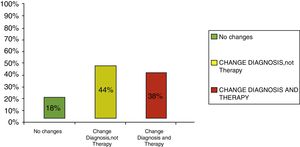
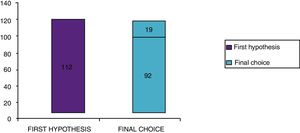
Clinical results in food allergyConcerning the 187 patients with diagnosis of food allergy, the implementation of CRD did not produce changes to the diagnostic-therapeutic choice in 33 of them (18%), while it entailed refining the diagnosis (D) or an overall change in diagnosis and therapy (D and T) respectively in 82 (44%) and in 72 (38%) patients (Fig. 6). With the traditional diagnostic method (first diagnostic-therapeutic hypothesis) a total of 112 self-injectable adrenaline devices were prescribed, whereas according to the molecular approach (final choice) such devices become 111 (including 19 adrenaline devices prescribed only after CRD), making it one unit less (Fig. 7). The statistical analysis of the correlation between the two diagnostic methods (traditional diagnostics vs. molecular approach) in identifying patients who were prescribed self-injectable adrenaline highlights an accord was observed between methods equal to 79% of cases; the agreement coefficient (k index) amounted to 0.56, meaning a moderate agreement, according to the interpretative scale of Landis and Koch.
The implementation of CRD made a change in diagnosis and therapy, i.e. the potential prescription and composition of AIT in 88 patients (32%). By statistically analyzing these data, the agreement coefficient (k index) of correlation between the two methods – traditional vs. molecular – in identifying patients who were prescribed AIT was 0.54, which is a moderate agreement; the results of CRD changed the clinical choice in about half of cases. This finding is consistent with previous studies. In fact, in the study by Sastre et al.39 an agreement between the AIT indication before and after the implementation of molecular diagnostics (Microarray ISAC) was found in 62 (46%) patients (kappa=0.1057±0.0413), meaning that after molecular diagnostics the therapeutic choice changed in as many as 54% of subjects. In the study on children (mostly polysensitized) by Stringari et al.,40 after CRD by Immunocap the previously established therapeutic choice (according to SPT) would have changed into prescription or different composition of AIT in 305 (47%) of 651 subjects. Also in the study by Passalacqua et al.38 on 318 polysensitized patients, the implementation of Microarray ISAC would have led to detecting in about 60% of patients at least one additional item of information, which consequently implied therapeutic adjustments. Lastly, Borghesan et al.,41 based on the discovery of sensitization to panallergens by molecular diagnostics, halted AIT prescription to 20 of 50 patients (40%). Our result confirms that there is a low agreement (though greater than in previous studies) concerning the potential clinical significance, in the AIT choice before and after molecular diagnostics. This emphasizes the usefulness of CRD in indicating the most appropriate therapy, at least in polysensitized patients. In this regard, in our real-life population, contrary to Sastre's,39 SPT sensitization to olive never led to AIT prescription at the first diagnostic-therapeutic hypothesis, since this pollen is not usually relevant from a clinical perspective in our latitudes (Northern Italy); this could have contributed to the slight increase of the degree of agreement between traditional diagnostics and CRD in our study. We also tried to evaluate if there could be a difference in the result obtained by dividing our population into two groups, 149 patients suffering from perennial allergy (those sensitized to dust mites and/or molds), compared to the 126 with seasonal allergy: according to the result of the chi-square test the difference was not significant. Regarding this aspect, Sastre et al.39 analyzed the differences between the main allergens, detecting changes in the rate of patients allocated to each group that, from a statistical point of view, could be translated into a variation of k index from “1” for olive and cypress to “<0.2” for all the others; anyhow, all allergens included were pollens, therefore seasonal allergens. Also Stringari et al.,40 using the same subdivision, detected variations in the percentage allocated to each pollen group. In any case, our study indicates that CRD is useful not only in patients suffering from pollen allergy, as highlighted for example by Sastre et al.,39 but also in patients sensitized to perennial allergens. In our population, 84 of 275 patients (31%) were sensitized to panallergens (profilin and/or polcacin), data apparently in line with the percentage of therapeutic change (32%). Similarly, in the pediatric study of Stringari et al.40 about 25% of patients were sensitized to profilin, in the pediatric population of Tripodi et al.45 about 20% of the 176 children sensitized to grass pollens had IgE for profilin while only four for polcacin, in the study by Borghesan41 on subjects older than 12 years profilin and/or polcacin were found in 40% of cases, in the adult population of Melioli et al.46 sensitization to profilin was present in 15% of patients and that for polcalcin in 5% of them, and lastly, in the study of Darsow on 101 adults with grass pollen allergy47 the sensitization to profilin and polcalcin were present respectively in nine and two patients. The percentage we found seems to confirm the literature data according to which profilin sensitization can mostly be found in the Mediterranean area rather than in Northern Europe, reason for which the impact of CRD on AIT prescription in those geographical areas could be less relevant, although profilin is not the sole significant factor in this aspect. In this regard, in our study profilin and/or polcacin were found in 38 (43%) of 88 patients in whom an overall change of diagnosis and therapy occurred. The other aspect is that in our study, using CRD, the AIT courses globally prescribed decreased from 339 to 283, precisely because in 56 cases there was not an actual sensitization to the genuine molecule (e.g., Phl p 1, Phl p 5, Bet v 1, Par j 2, Der p 1, Der p 2, Amb a 1), which was erroneously apparent by SPT and symptomatology; we would have then prescribed 56 AIT to subjects not truly allergic to that specific source and that would therefore result in an ineffective treatment. In this regard, in the study conducted by Stringari et al.,40 an actual sensitization to the respective genuine allergens was lacking in a significant percentage of patients with a supposed relevant clinical allergy, more specifically in 464 reactions on a total of 1716 (27%) taking into consideration all pollens, and IgE to profilin and polcacin (or both) were relevant in justifying 173 (37%) of these 464 reactions to SPT. In Sastre's paper,39 the lack of sensitization to genuine molecules was found in 116 reactions to SPT out of a total of 530 (22%), taking into consideration all pollens. Borghesan et al.41 stopped AIT prescription in 20 of 50 patients, because they resulted sensitized to panallergens, regardless of the presence or absence of sensitization to genuine molecules. In fact, it seems that such patients would find AIT less effective, due to insufficient presence of panallergens in therapeutic extracts, which are notoriously standardized on genuine allergens; in any case, this aspect has not been addressed in our work, while other clinical studies established to confirm or remove this suspect, are underway. At the same time, we need to specify that in our study, on 283 AIT courses globally prescribed according to the molecular approach, in 26 cases the sensitization was actually confirmed as clinically relevant (genuine) only after molecular diagnostics; regarding this aspect, in the study of Sastre39 in 175 negative outcomes to SPT a genuine sensitization was found in 56 of them. Moving on to the economic issue, in our study the implementation of CRD entailed a total saving of €42,250 on 275 patients. This confirms that not only is CRD effective in improving the selection of patients who can benefit from AIT, the only anti allergic treatment with both a disease-modifying and a proven cost-effective profile29–31 compared to symptomatic drug treatment, but also decreases the overall costs, from both the individual and the health care system perspective. The study by Borghesan et al.41 also evaluated the economic aspect of a diagnostic approach through SPT or molecular diagnostics, both including diagnostics and AIT treatment; an overall saving of €18,538 on 50 patients was detected. Also the study conducted by Lepage-Nefkens et al.42 in 2015 showed how, beyond increasing full responders to AIT [from 9422 (95%CI; 7384–11,891) to 13,239 (95%CI; 10,039–16,868)], CRD would provide a greater saving on the total costs expected for each patient, that would change from €7555 (95%CI; €7496–€7618) to €7255 (95%CI; €7062–€7453). It can be concluded that the detection of specific IgE to recombinant molecules, despite appearing more expensive compared with traditional tests, produces more reliable information in merit of AIT prescription, thus resulting in a significant reduction of global costs backed by the health care system. In any case, as previously discussed, further studies are warranted to verify the profile of greater clinical efficacy of AIT prescribed according to the molecular approach.
Food allergyConcerning food allergy, the implementation of CRD entailed an overall change of diagnosis and therapy (namely the potential prescription and the type of avoidance diets as well as the potential prescription of self-injectable adrenaline) in 82 (38%) patients. Statistically analyzing self-injectable adrenaline, the agreement coefficient (k index) of correlation between the two methods – traditional vs. molecular – in identifying patients to whom prescribe was 0.56, i.e. a moderate agreement; this indicates that molecular diagnostics changes the clinical choice in about half of cases. Such a result is very similar to that obtained in respiratory allergy concerning AIT prescription. In the only study available thus far,43 performed on an adult population in real-life, there was a fair degree of agreement between the decision of the specialist and the adrenaline prescription influenced by Microarray (59 of 86 patients, that is 68.6%, with a k index 0.372 (95% CI: 0.185–0.559; p<0.001). Our result therefore confirms the quite low agreement (although slightly greater compared to the study mentioned above), concerning the potential clinical significance when choosing the self-injectable adrenaline before and after using molecular diagnostics, thus emphasizing its real usefulness in indicating the severity of clinical risk, at least in those patients with complex sensitizations. However, the small difference found between the two areas – respiratory and food allergy – could lead one to say that the lack of using molecular diagnostics makes it easier to make mistakes when prescribing AIT rather than prescribing adrenaline. In our study, adrenaline devices prescribed after CRD have decreased from 112 to 111, therefore with a loss of 1 unit and a saving of €80. At the same time it is important to highlight that, of 111 adrenaline devices prescribed according to the molecular approach, in 19 cases the sensitization was confirmed as clinically significant and potentially dangerous only after molecular diagnostics was carried out: it was mostly about hazelnut (Cor a 14) and wheat (gliadin) allergies, which had been underestimated. Actually, even though the global data between the two approaches seem to be overlapping, one should reason on the usefulness of CRD in improving the diagnostic-therapeutic appropriateness. The adrenaline prescription does not depend only on the severity of the clinical reaction, but also on the type of sensitizing molecule. A mild clinical reaction may induce a “non-prescription” of adrenaline, underestimating the risk of anaphylaxis in case of sensitization to stable molecules, which could potentially cause anaphylaxis, especially when cofactors are present48,49; on the other hand, a mild/moderate clinical reaction to an ubiquitous source, which could potentially cause anaphylaxis, such as hazelnut, may induce an excess of adrenaline prescription. Therefore, globally a prescription occurs in approximately the same number. In our study we have refined the diagnosis through a better definition of the triggering allergen(s), and/or we have recommended a specific diet with the potential prescription of self-injectable adrenaline in 117 patients, which were actually found to be sensitized to molecules with a high risk of severe reactions (in 68% LTPs). Therefore, a more precise diagnosis enables to better define the foods to be avoided and thus prevent future relapses, which may occur for an incorrect definition of the avoidance diet but possibly also because of an association of a cofactor. In fact, as already known, at the base of reactions triggered by cofactors there is very frequently an LTP allergen, therefore a patient with known LTP sensitization should be informed of the risk he/she could face in case of association with one or more cofactors when ingesting the specific responsible food.49 In any case, being aware that a patient, possibly evaluated at low-medium risk of severe reaction according to clinical manifestation, has however a potentially serious underlying sensitization, leads to following him/her with greater attention, for example in evaluating the possible future development of reactions to foods cross-reactive with the triggering one, as often happens with LTPs. By contrast, in 11 patients CRD has clarified that the avoidance diet was not necessary, given the absence of sensitization to potentially dangerous molecules. This patient category is actually particularly common in real life, probably more than it appears in our case series: it concerns subjects who undergo an avoidance diet on the basis of a SPT finding, possibly for several years, when they actually had only experienced a mild clinical reaction.
ConclusionsCurrently, allergic diseases are often underrated, thus costs are mostly and primarily paid by the patients and their families. In addition, allergies particularly affect learning and performance, both in school and work place, causing high costs for the whole society. All this cannot be precisely quantified from the economical point of view but has a relevant influence in a modern society.44 An improvement in the diagnosis and management of such diseases, based on the most recent guidelines, could provide significant savings. In this context, CRD plays a notable role, since, as statistically confirmed by our real-life study, in about 50% of cases it involves a change in the therapeutic choice. In particular, it allows to: (1) distinguish genuine vs. cross-reactive sensitization in polysensitized patients, improving the knowledge of the real triggering allergens; (2) advise patients to avoid the intake of food and/or the exposure to inhalant agents that show cross-reactivity, because of the presence of panallergens; predicting the severity of the allergic reaction in case of consumption of a specific food to which one is sensitized; (3) prescribe self-injectable adrenaline devices to patients most at risk of severe anaphylaxis; (4) identify with greater accuracy the suitable patient for AIT, for whom this treatment will prove to be more effective and safe. The financial impact of misdiagnosis could be addressed assessing serious harm to patients (death or permanent disability) for wrong AIT prescription, costs of asthma medications, costs of testing to confirm diagnosis of asthma, costs of emergency room visits, and the cost for specialist consultations. Studies including such issues are likely to result in a higher economic advantage
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
Authors’ contributions- •
Study conception and design: Peveri, Montagni, Savi
- •
Acquisition of data: Peveri, Pattini, Costantino, Montagni, Roncallo, Villalta, Savi
- •
Analysis and interpretation of data: Peveri, Montagni, Savi
- •
Drafting of manuscript: Pattini, Incorvaia, Montagni, Savi
- •
Critical revision: Incorvaia, Savi
All authors read and approved the final manuscript.
Conflict of interestThe authors have no conflict of interest to declare.