The del22q11 syndrome patients present immunological abnormalities associated to thymus alterations. Up to 75% of them present cardiopathies and thymus is frequently removed during surgery. The thymectomy per se has a deleterious effect concerning lymphocyte subpopulations, and T cell function. When compared to healthy controls, these patients have higher infections propensity of variable severity. The factors behind these variations are unknown. We compared immunological profiles of del22q11.2 Syndrome patients with and without thymectomy to establish its effect in the immune profile.
MethodsForty-six del22q11.2 syndrome patients from 1 to 16 years old, 19 of them with partial or total thymectomy were included. Heart disease type, heart surgery, infections events and thymus resection were identified. Immunoglobulin levels, flow cytometry for lymphocytes subpopulations and TREC levels were determined, and statistical analyses were performed.
ResultsThe thymectomy group had a lower lymphocyte index, both regarding total cell count and when comparing age-adjusted Z scores. Also, CD3+, CD4+ and CD8+ lower levels were observed in this group, the lowest count in those patients who had undergone thymus resection during the first year of life. Their TREC level median was 23.6/μL vs 16.1μL in the non-thymus group (p=0.22). No differences were identified regarding immunoglobulin levels or infection events frequencies over the previous year.
ConclusionPatients with del22q11.2 syndrome subjected to thymus resection present lower lymphocyte and TREC indexes when compared to patients without thymectomy. This situation may be influenced by the age at the surgery and the time elapsed since the procedure.
The aplasia, hypoplasia, and abnormal cell migration of the thymus gland affects the T lymphocyte differentiation and the selection process responsible for cellular immunity.1–4 This process is compromised in primary congenital immunodeficiencies, or specific syndromes such as the Velocardiofacial or deletion 22q11.2 syndrome (MIM #192430; VCFS, del22q11 syndrome, del22q11S). The del22q11S is present in 1:2000 to 1:4000 live borns,5 and is caused by a heterozygous 1.5–3.0 Mb microdeletion in the 22q11.2 chromosome.6–8 The del22q11S phenotype is highly variable, affecting facial, velopharyngeal, cardiac, and immunological areas, among others. The immune alterations in del22q11S range from mild to severe and are secondary to a spectrum of thymus anomalies such as hypoplasia (the most frequent clinical presentation), ectopic tissue present mostly on the neck; or aplasia present in 0.5% of the patients, leading to severe immunodeficiency.9,10
The del22q11S region includes approximately 40 genes; both thymus and conotruncal heart defects result from genes affected by the microdeletion, including TBX1 (MIM 602054), CRKL (MIM 602007), and ERK2 (MIM 176948) genes, among others.11–13TBX1, the most studied of this group of genes, encodes a transcription factor protein that contains a “T-box” domain, which directly activates to myogenic factor 5 (MYF5), myogenic differentiation factor 1 (MYOD1) and fibroblast growth factors 8 and 10 (FGF8, FGF10), which are involved in the development of the thymus gland and play a crucial role in its migration.14,15
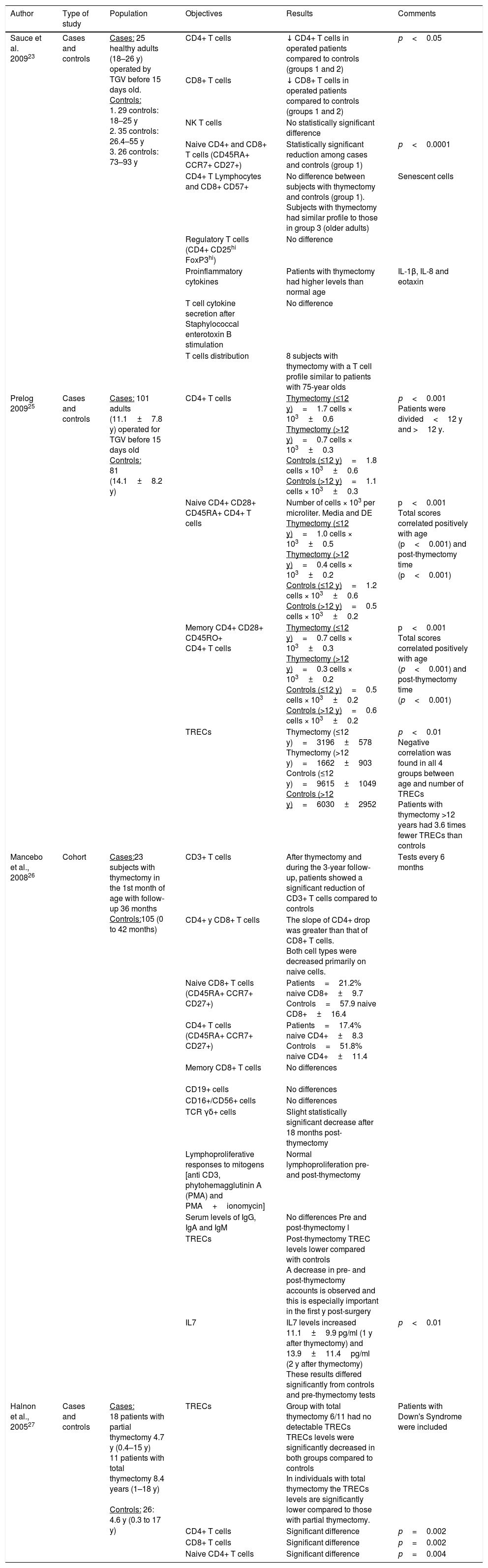
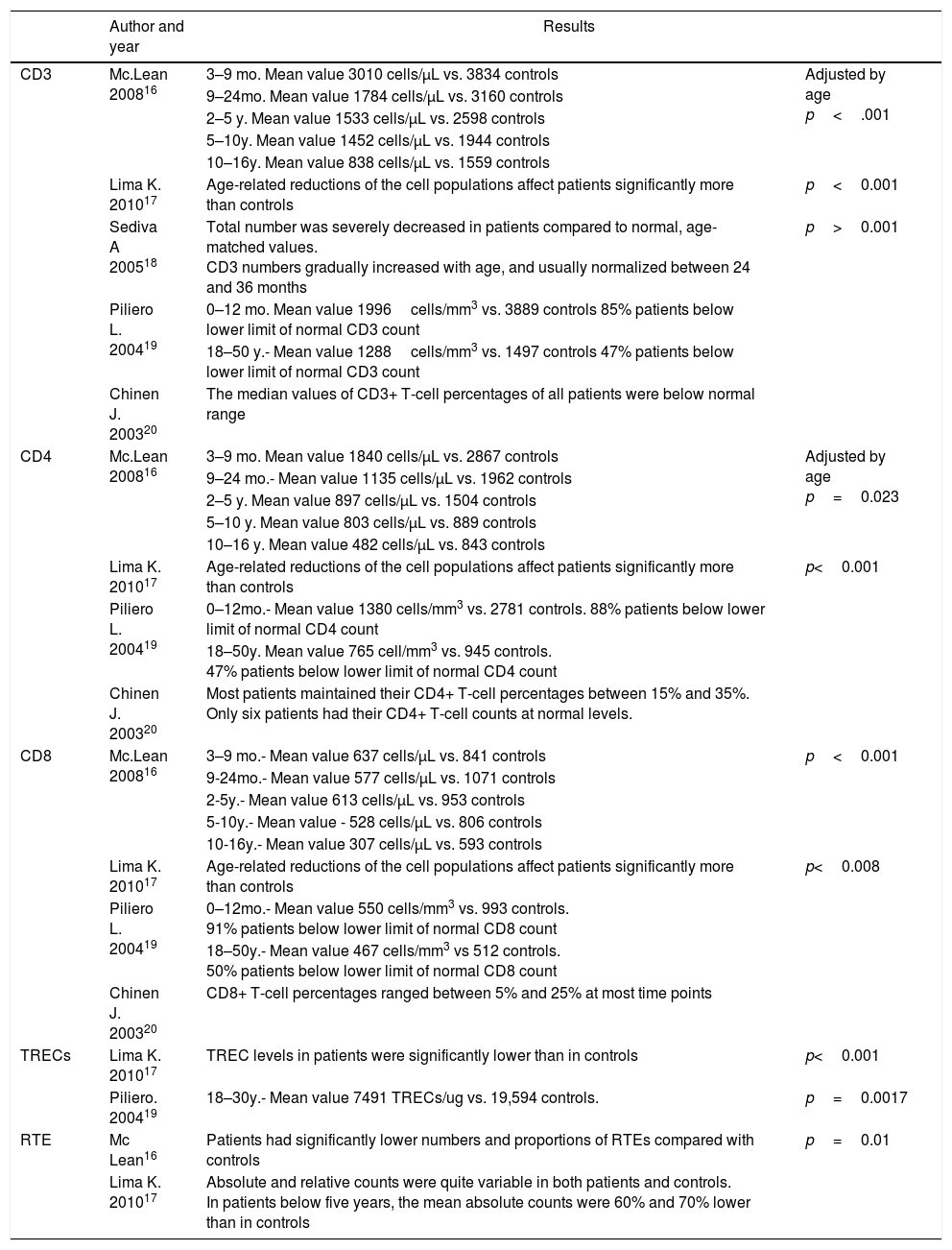
Several studies have described immunological abnormalities in del22q11S patients, including lower percentages and total T cell subpopulations counts,16–20 lower levels in T cell receptor excision circles (TREC)17–19 and recent thymic emigrants (RTE).16,17 (see Table E1). Up to 75% of del22q11S patients present congenital heart disease,21,22 therefore cardiovascular surgery is commonly performed in them, frequently with an anterior chest surgical procedure, as it allows direct visualization of the superior mediastinum structures. In this surgical approach, the thymus gland localization prevents an adequate visualization of the cardiac structures, hence its removal in many cases.23,24 It has also been demonstrated that in patients with isolated heart disease, the thymectomy procedure per se has a deleterious effect concerning lymphocyte subpopulations, and T cell function (Table 1).23,25–29
Demographic data and lymphocyte populations in patients with 22q11.2 deletion syndrome.
| Author and year | Results | ||
|---|---|---|---|
| CD3 | Mc.Lean 200816 | 3–9 mo. Mean value 3010 cells/μL vs. 3834 controls | Adjusted by age p<.001 |
| 9–24mo. Mean value 1784 cells/μL vs. 3160 controls | |||
| 2–5 y. Mean value 1533 cells/μL vs. 2598 controls | |||
| 5–10y. Mean value 1452 cells/μL vs. 1944 controls | |||
| 10–16y. Mean value 838 cells/μL vs. 1559 controls | |||
| Lima K. 201017 | Age-related reductions of the cell populations affect patients significantly more than controls | p<0.001 | |
| Sediva A 200518 | Total number was severely decreased in patients compared to normal, age-matched values. CD3 numbers gradually increased with age, and usually normalized between 24 and 36 months | p>0.001 | |
| Piliero L. 200419 | 0–12 mo. Mean value 1996cells/mm3 vs. 3889 controls 85% patients below lower limit of normal CD3 count | ||
| 18–50 y.- Mean value 1288cells/mm3 vs. 1497 controls 47% patients below lower limit of normal CD3 count | |||
| Chinen J. 200320 | The median values of CD3+ T-cell percentages of all patients were below normal range | ||
| CD4 | Mc.Lean 200816 | 3–9 mo. Mean value 1840 cells/μL vs. 2867 controls | Adjusted by age p=0.023 |
| 9–24 mo.- Mean value 1135 cells/μL vs. 1962 controls | |||
| 2–5 y. Mean value 897 cells/μL vs. 1504 controls | |||
| 5–10 y. Mean value 803 cells/μL vs. 889 controls | |||
| 10–16 y. Mean value 482 cells/μL vs. 843 controls | |||
| Lima K. 201017 | Age-related reductions of the cell populations affect patients significantly more than controls | p<0.001 | |
| Piliero L. 200419 | 0–12mo.- Mean value 1380 cells/mm3 vs. 2781 controls. 88% patients below lower limit of normal CD4 count | ||
| 18–50y. Mean value 765 cell/mm3 vs. 945 controls. 47% patients below lower limit of normal CD4 count | |||
| Chinen J. 200320 | Most patients maintained their CD4+ T-cell percentages between 15% and 35%. Only six patients had their CD4+ T-cell counts at normal levels. | ||
| CD8 | Mc.Lean 200816 | 3–9 mo.- Mean value 637 cells/μL vs. 841 controls | p<0.001 |
| 9-24mo.- Mean value 577 cells/μL vs. 1071 controls | |||
| 2-5y.- Mean value 613 cells/μL vs. 953 controls | |||
| 5-10y.- Mean value - 528 cells/μL vs. 806 controls | |||
| 10-16y.- Mean value 307 cells/μL vs. 593 controls | |||
| Lima K. 201017 | Age-related reductions of the cell populations affect patients significantly more than controls | p<0.008 | |
| Piliero L. 200419 | 0–12mo.- Mean value 550 cells/mm3 vs. 993 controls. 91% patients below lower limit of normal CD8 count | ||
| 18–50y.- Mean value 467 cells/mm3 vs 512 controls. 50% patients below lower limit of normal CD8 count | |||
| Chinen J. 200320 | CD8+ T-cell percentages ranged between 5% and 25% at most time points | ||
| TRECs | Lima K. 201017 | TREC levels in patients were significantly lower than in controls | p<0.001 |
| Piliero. 200419 | 18–30y.- Mean value 7491 TRECs/ug vs. 19,594 controls. | p=0.0017 | |
| RTE | Mc Lean16 | Patients had significantly lower numbers and proportions of RTEs compared with controls | p=0.01 |
| Lima K. 201017 | Absolute and relative counts were quite variable in both patients and controls. In patients below five years, the mean absolute counts were 60% and 70% lower than in controls | ||
Mc Lean.- 27 patients and 54 controls (3mo–16y), Lima.- 43 patients and 24 controls (1y–54y), Sediva.- 34 patients (4d–19y), Piliero.- 409 patients and 103 controls, Chinen.- 30 patients. Mo., months; y, years; d, days.
Immune alterations in patients with congenital heart disease and incidental thymectomy.
| Author | Type of study | Population | Objectives | Results | Comments |
|---|---|---|---|---|---|
| Sauce et al. 200923 | Cases and controls | Cases: 25 healthy adults (18–26 y) operated by TGV before 15 days old. Controls: 1. 29 controls: 18–25 y 2. 35 controls: 26.4–55 y 3. 26 controls: 73–93 y | CD4+ T cells | ↓ CD4+ T cells in operated patients compared to controls (groups 1 and 2) | p<0.05 |
| CD8+ T cells | ↓ CD8+ T cells in operated patients compared to controls (groups 1 and 2) | ||||
| NK T cells | No statistically significant difference | ||||
| Naive CD4+ and CD8+ T cells (CD45RA+ CCR7+ CD27+) | Statistically significant reduction among cases and controls (group 1) | p<0.0001 | |||
| CD4+ T Lymphocytes and CD8+ CD57+ | No difference between subjects with thymectomy and controls (group 1). Subjects with thymectomy had similar profile to those in group 3 (older adults) | Senescent cells | |||
| Regulatory T cells (CD4+ CD25hi FoxP3hi) | No difference | ||||
| Proinflammatory cytokines | Patients with thymectomy had higher levels than normal age | IL-1β, IL-8 and eotaxin | |||
| T cell cytokine secretion after Staphylococcal enterotoxin B stimulation | No difference | ||||
| T cells distribution | 8 subjects with thymectomy with a T cell profile similar to patients with 75-year olds | ||||
| Prelog 200925 | Cases and controls | Cases: 101 adults (11.1±7.8 y) operated for TGV before 15 days old Controls: 81 (14.1±8.2 y) | CD4+ T cells | Thymectomy (≤12 y)=1.7 cells × 103±0.6 Thymectomy (>12 y)=0.7 cells × 103±0.3 Controls (≤12 y)=1.8 cells × 103±0.6 Controls (>12 y)=1.1 cells × 103±0.3 | p<0.001 Patients were divided<12 y and >12 y. |
| Naive CD4+ CD28+ CD45RA+ CD4+ T cells | Number of cells × 103 per microliter. Media and DE Thymectomy (≤12 y)=1.0 cells × 103±0.5 Thymectomy (>12 y)=0.4 cells × 103±0.2 Controls (≤12 y)=1.2 cells × 103±0.6 Controls (>12 y)=0.5 cells × 103±0.2 | p<0.001 Total scores correlated positively with age (p<0.001) and post-thymectomy time (p<0.001) | |||
| Memory CD4+ CD28+ CD45RO+ CD4+ T cells | Thymectomy (≤12 y)=0.7 cells × 103±0.3 Thymectomy (>12 y)=0.3 cells × 103±0.2 Controls (≤12 y)=0.5 cells × 103±0.2 Controls (>12 y)=0.6 cells × 103±0.2 | p<0.001 Total scores correlated positively with age (p<0.001) and post-thymectomy time (p<0.001) | |||
| TRECs | Thymectomy (≤12 y)=3196±578 Thymectomy (>12 y)=1662±903 Controls (≤12 y)=9615±1049 Controls (>12 y)=6030±2952 | p<0.01 Negative correlation was found in all 4 groups between age and number of TRECs Patients with thymectomy >12 years had 3.6 times fewer TRECs than controls | |||
| Mancebo et al., 200826 | Cohort | Cases:23 subjects with thymectomy in the 1st month of age with follow-up 36 months Controls:105 (0 to 42 months) | CD3+ T cells | After thymectomy and during the 3-year follow-up, patients showed a significant reduction of CD3+ T cells compared to controls | Tests every 6 months |
| CD4+ y CD8+ T cells | The slope of CD4+ drop was greater than that of CD8+ T cells. Both cell types were decreased primarily on naive cells. | ||||
| Naive CD8+ T cells (CD45RA+ CCR7+ CD27+) | Patients=21.2% naive CD8+±9.7 Controls=57.9 naive CD8+±16.4 | ||||
| CD4+ T cells (CD45RA+ CCR7+ CD27+) | Patients=17.4% naive CD4+±8.3 Controls=51.8% naive CD4+±11.4 | ||||
| Memory CD8+ T cells | No differences | ||||
| CD19+ cells | No differences | ||||
| CD16+/CD56+ cells | No differences | ||||
| TCR γδ+ cells | Slight statistically significant decrease after 18 months post-thymectomy | ||||
| Lymphoproliferative responses to mitogens [anti CD3, phytohemagglutinin A (PMA) and PMA+ionomycin] | Normal lymphoproliferation pre- and post-thymectomy | ||||
| Serum levels of IgG, IgA and IgM | No differences Pre and post-thymectomy l | ||||
| TRECs | Post-thymectomy TREC levels lower compared with controls A decrease in pre- and post-thymectomy accounts is observed and this is especially important in the first y post-surgery | ||||
| IL7 | IL7 levels increased 11.1±9.9 pg/ml (1 y after thymectomy) and 13.9±11.4pg/ml (2 y after thymectomy) These results differed significantly from controls and pre-thymectomy tests | p<0.01 | |||
| Halnon et al., 200527 | Cases and controls | Cases: 18 patients with partial thymectomy 4.7 y (0.4–15 y) 11 patients with total thymectomy 8.4 years (1–18 y) Controls: 26: 4.6 y (0.3 to 17 y) | TRECs | Group with total thymectomy 6/11 had no detectable TRECs TRECs levels were significantly decreased in both groups compared to controls In individuals with total thymectomy the TRECs levels are significantly lower compared to those with partial thymectomy. | Patients with Down's Syndrome were included |
| CD4+ T cells | Significant difference | p=0.002 | |||
| CD8+ T cells | Significant difference | p=0.002 | |||
| Naive CD4+ T cells | Significant difference | p=0.004 |
y, years; TGV, Transposition of the great vessels.
When compared to healthy controls, patients with del22q11S have a higher propensity to infections, mainly concerning the upper airways, probably associated to thymus alterations, and showing differences in severity. The factors behind these clinical variations are unknown, one of them may be the damaging effect of the incidental thymectomy, however this situation has not been analyzed in depth, and as far as we known there is only one study that included three del22q11S patients that addressed this aspect. The objective of our study was to analyze and compare the immunological profiles of del22q11.2S patients with complete or partial thymectomy, and patients without thymus surgical resection, in order to establish the effect of the thymectomy procedure in the immune profile of these patients.
Materials and methodsFemale and male Mexican mestizo patients from 1 to 16 years old with del22q11S diagnosis confirmed by FISH analysis (TUPLE I probe; Vysis®, Abbott Laboratories, Abbott Park, IL, USA) from the “del22q11 Clinic” at the Hospital Infantil de México Federico Gómez (HIMFG) were included with informed consent in a cross-sectional study. The research board of our institution approved the study (HIM/2012/023). A clinical evaluation was carried out to identify the type of heart disease, heart surgery and findings or interventions during the procedure, thymus visualization and whether it was resected either partially or totally. Also, upper respiratory tract infections (URTI), otitis, gastroenteritis and pneumonia frequency over the previous year was registered, as well as the number of hospitalizations due to infections. Thymus ultrasound analysis was carried out with a 10.4-MHz linear transducer (Antares, Siemens®), the patients were in supine position with a pillow behind their shoulders, using a suprasternal window.
The immunological profiles of the patients were established with a complete blood count, immunoglobulin levels, flow cytometry for lymphocytes subpopulations and PCR for TREC levels.
Serum immunoglobulin levels (IgG, IgA and IgM) were measured by nephelometry (BN Prospec; Siemens, Munich, Germany).
Whole blood cells were stained with the corresponding monoclonal antibodies to determine the percentage and the absolute number of the following lymphocyte populations: CD3+, CD3+ CD4+, CD3+ CD8+, CD16+ CD56+ CD3−, and CD3+ CD19+ in BD FACSCanto II flow cytometer (Becton Dickinson, CA, USA).
TREC levels were measured with a technique adapted for Douek et al.30 in a filter paper whole blood sample; for DNA extraction, two 0.3cm pinpricks were punctured in a Guthrie card (AxyPrep Multisource Genomic DNA Miniprep Kit). The DNA obtained was resuspended in 100μL of the elution buffer, 5μL of each DNA was used to quantify TREC levels and Ribonucleasa P (RnasaP), by means of duplex reaction. For the standard curves, serial dilutions of standard plasmid with known concentrations of signal joint TREC (sjTREC) sequences were used, and for RnasaP (Applied Biosystems Human DNA and Copy Reference Assay Kits). The final measurement values were obtained from extrapolating average values of the Threshold Cycle (Ct) of each sample on each blank sequence's calibration curves.
Patients with uncontrolled heart conditions, concomitant blood diseases, and/or immunosuppressive treatment, systemic steroids, or drugs that influence leucocyte or erythrocyte indexes or affect bone marrow, were excluded from the study. The participant investigators were blinded about the results during the analysis.
The patients were divided into two groups, group 1 included patients without heart surgery and whose thymus gland was identified by ultrasound analysis; group 2 included patients who had undergone heart surgery and have been subject to partial or total thymectomy confirmed through medical records and ultrasound reports. The thymectomies were carried out only to allow a better visualization during heart surgery. The presence of ectopic thymic tissue could not be ruled out.
The comparison between lymphocyte indexes was carried out using total cell counts as well as Z score calculations adjusted to normal values for the age group.31 Mann–Whitney U test was applied to compare leucocyte indexes, lymphocyte subpopulations, immunoglobulin and TREC levels between both groups. An x2 analysis was used to compare the infectious processes, and SPSS version 21 was used for statistical analysis.
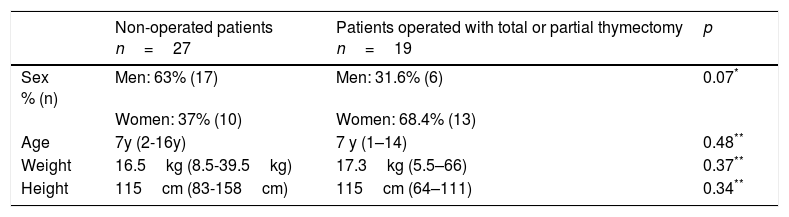
ResultsA total of 46 patients (23 female and 23 males): 27 patients without a heart surgery history and thymus gland evidenced by ultrasound and 19 patients with the antecedent of heart surgery and evidence of partial or total thymectomy, were included in our study. The median patients age was seven years old with a range from 1 to 16 years. There were no significant differences between age, sex, weight and height between both compared groups (Table 2). Ten patients (10/46; 21.7%) had a healthy heart and 36 (36/46; 78.3%) presented some type of congenital heart disease; 17 of them had not undergone surgery and 19 had. The most frequently observed congenital heart disease was Tetralogy of Fallot (7/46; 15.2%), and truncus arteriosus (7/46; 15.2%). The median age at the time of surgery was 1 year and 11 months with a minimum of 20 days and a maximum of 7 years. The median for the time period elapsed between surgery and patient study inclusion was 5.5 years (from 8 months to 12 years).
Demographic and anthropometric characteristics of patients included in the study.
| Non-operated patients n=27 | Patients operated with total or partial thymectomy n=19 | p | |
|---|---|---|---|
| Sex % (n) | Men: 63% (17) Women: 37% (10) | Men: 31.6% (6) Women: 68.4% (13) | 0.07* |
| Age | 7y (2-16y) | 7 y (1–14) | 0.48** |
| Weight | 16.5kg (8.5-39.5kg) | 17.3kg (5.5–66) | 0.37** |
| Height | 115cm (83-158cm) | 115cm (64–111) | 0.34** |
y, years; n, number of patients.
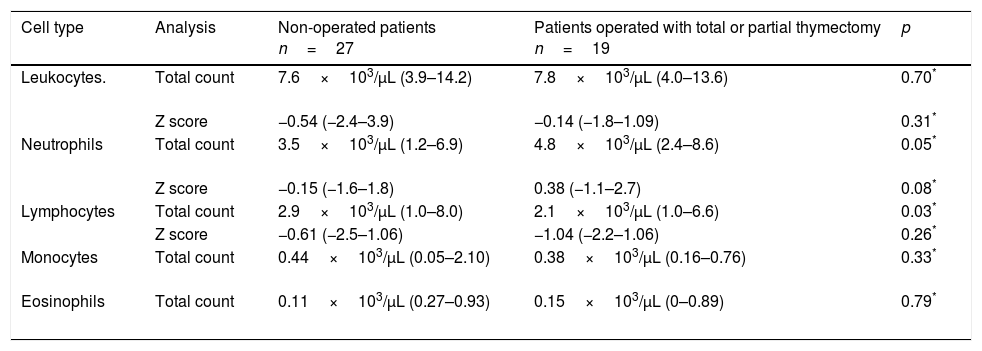
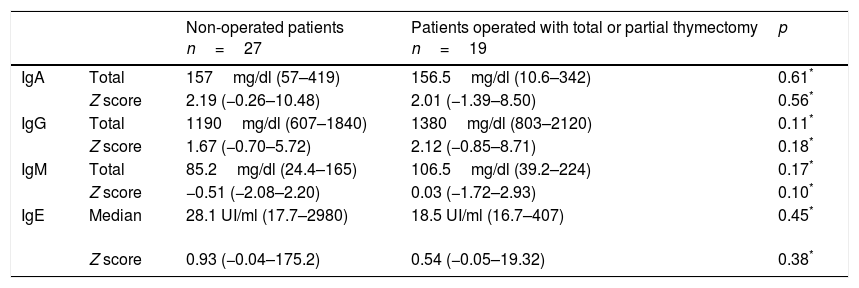
Median leucocyte counts, immunoglobulin indexes, and age-adjusted Z scores31are shown in Tables 3 and 4 for both groups. The thymectomy group showed a lower lymphocyte index when compared to the patients without surgery events, both regarding the total cell count as well as when comparing age-adjusted Z scores. No significant differences were found between groups regarding immunoglobulin levels.
White blood cells counts in both groups and their comparison31
| Cell type | Analysis | Non-operated patients n=27 | Patients operated with total or partial thymectomy n=19 | p |
|---|---|---|---|---|
| Leukocytes. | Total count | 7.6×103/μL (3.9–14.2) | 7.8×103/μL (4.0–13.6) | 0.70* |
| Z score | −0.54 (−2.4–3.9) | −0.14 (−1.8–1.09) | 0.31* | |
| Neutrophils | Total count | 3.5×103/μL (1.2–6.9) | 4.8×103/μL (2.4–8.6) | 0.05* |
| Z score | −0.15 (−1.6–1.8) | 0.38 (−1.1–2.7) | 0.08* | |
| Lymphocytes | Total count | 2.9×103/μL (1.0–8.0) | 2.1×103/μL (1.0–6.6) | 0.03* |
| Z score | −0.61 (−2.5–1.06) | −1.04 (−2.2–1.06) | 0.26* | |
| Monocytes | Total count | 0.44×103/μL (0.05–2.10) | 0.38×103/μL (0.16–0.76) | 0.33* |
| Eosinophils | Total count | 0.11×103/μL (0.27–0.93) | 0.15×103/μL (0–0.89) | 0.79* |
Serum immunoglobulin levels in both groups and their comparison31.
| Non-operated patients n=27 | Patients operated with total or partial thymectomy n=19 | p | ||
|---|---|---|---|---|
| IgA | Total | 157mg/dl (57–419) | 156.5mg/dl (10.6–342) | 0.61* |
| Z score | 2.19 (−0.26–10.48) | 2.01 (−1.39–8.50) | 0.56* | |
| IgG | Total | 1190mg/dl (607–1840) | 1380mg/dl (803–2120) | 0.11* |
| Z score | 1.67 (−0.70–5.72) | 2.12 (−0.85–8.71) | 0.18* | |
| IgM | Total | 85.2mg/dl (24.4–165) | 106.5mg/dl (39.2–224) | 0.17* |
| Z score | −0.51 (−2.08–2.20) | 0.03 (−1.72–2.93) | 0.10* | |
| IgE | Median | 28.1 UI/ml (17.7–2980) | 18.5 UI/ml (16.7–407) | 0.45* |
| Z score | 0.93 (−0.04–175.2) | 0.54 (−0.05–19.32) | 0.38* |
IgA, Immunoglobulin A; IgG, Immunoglobulin G; IgM, Immunoglobulin M; IgE, Immunoglobulin E.
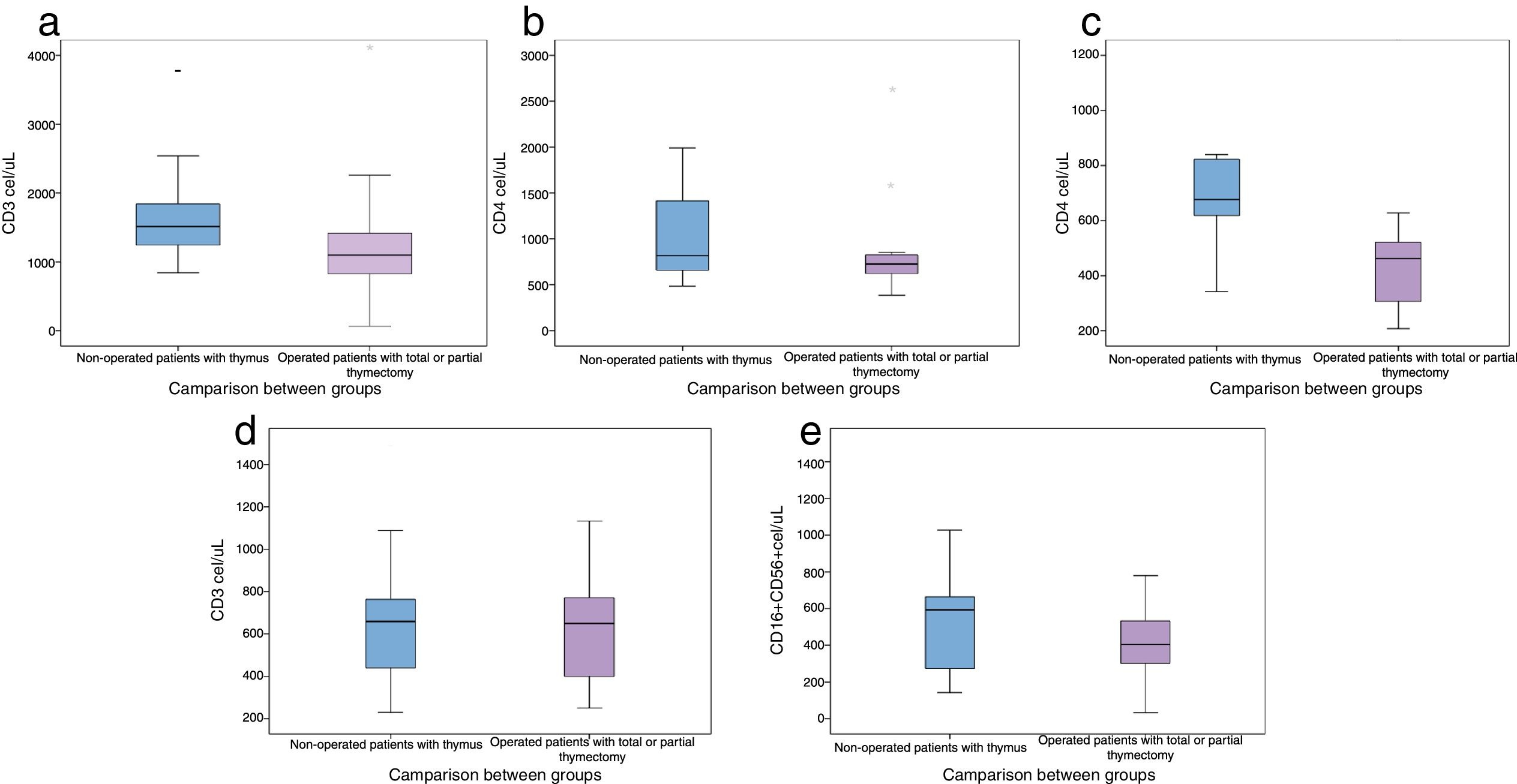
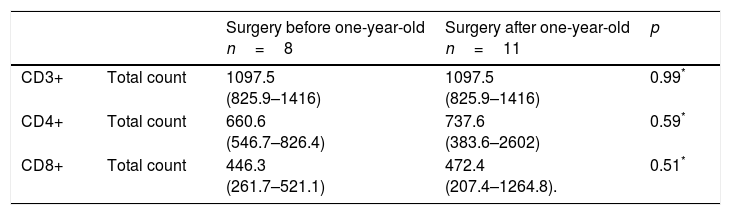
When comparing lymphocyte subpopulations, lower levels of CD3+, CD4+ and CD8+ were observed in the post-surgical thymectomy group (Fig. 1). The TREC level median was 23.6/μL (0–60μL) for the group with a thymus gland vs. 16.1μL (2.4–46.3μL) in the non-thymus group (p=0.22). An analysis was carried out to compare the ages in which thymectomies had been performed and the lymphocyte subpopulation counts at the time of the study; the lowest count of these subpopulations was present in those patients who had undergone thymus resection during their first year of life (Table 5).
Comparison of lymphocyte subpopulations levels in patients operated before and after 1-year-old.
There was a negative Spearman correlation between the months lapsed since surgery and the total lymphocyte counts in the group that had undergone surgery, with a r=−0.50 (p=0.03); total neutrophils: r=−0.27 (p=0.26); total lymphocytes: r=−0.33 (p=0.17); total CD3+: r=−0.19 (p=0.49); total CD4+: r=−0.25 (p=0.35); total CD8+: r=−0.10 (p=0.70), and TRECs: r=−0.74 (p=0.005) values.
A total of 41 hospitalizations due to infection events mostly associated to lower respiratory tract infections were registered; 29 of them corresponded to the intact thymus gland patients group, the hospitalization events average was 1.07 vs. 0.67 in the thymus gland group vs. the thymectomy group, with a p=0.41. The URTI median in the thymectomy group was 2 vs. 3 in the thymus group, p=0.52; no differences were found between groups regarding the frequency of otitis, sinusitis and gastrointestinal tract infections reported over the last year. The infectious diseases median over the last year, whether for URTI, otitis, sinusitis or gastrointestinal tract infections was four in both groups.
DiscussionA high percentage of del22q11S patients present some type of congenital heart disease, and need a surgical correction.21,22 Up to 50% of the children subject to heart surgery, have incidental thymus resection in order to increase exposure of the surgical field,23 therefore a probable effect of the thymectomy could be a decreased lymphopoiesis that would alter the host ability to generate new T cells compromising their immune response.32,33 Our study includes the highest number of patients diagnosed with del22q11.2S so far regarding thymectomy and its effects in their immunological profile.
In this study a similar leucocytes total count was observed in both groups of del22q11.2S patients, however, the total count of lymphocytes was lower in the thymectomy group, albeit within the normal reference range. As has been previously reported,34–36 we identified lower CD3+, CD4+ and CD8+ lymphocyte levels, although this was not the case for B and NK lymphocytes.
The reduction in lymphocyte counts regarding the time lapse after surgery has been little studied, and although some authors have reported similar lymphocyte subpopulation levels in patients who had undergone thymectomies and those who had not, with a five-year time lapse37; other authors disagree.38 In our study, the reduction in lymphocyte counts was exacerbated in children who had undergone thymus resection surgery before one year of age, furthermore, these levels had not reached normal values even five years after surgery.
Regarding TREC levels, we identified a tendency of lower values in our two groups of patients (with or without thymectomy) than the values reported for healthy populations,39,40 a situation that is in agreement with their diagnosis of del22q11S. In some reports, differences have been observed in TREC levels when comparing patients with or without thymectomy, in the sense that patients who preserved part of the thymic tissue had decreased TREC levels, albeit in a lower proportion that those patients who underwent total resection.27 This observation supports the hypothesis that there are secondary immune alterations associated to incidental thymectomies, and this is particularly reflected in lymphocyte counts and TREC levels.
We did not observe an increase in the number of infections or hospitalizations events for the group of patients with total thymus resection, however, it is worth considering that the longest time lapse between surgery and the study inclusion was 12 years, and although there were not short-term direct clinical consequences observed in our study, we cannot be certain whether there would be long-term repercussions. It is known that the thymus undergoes age-related involution, however its mass can grow when immune reconstitution is necessary,37 as occurs for example in HIV patients after antiretroviral drug treatment, and in patients after myeloablative chemotherapy. Therefore, it should be considered that a thymus resection during cardiothoracic surgery in del22q11S patients could decrease the possibility of an immune reconstitution event.
Although the infection data in our study was collected retrospectively, the results shown in this report suggest that the impact of thymectomy in early childhood in del22q11S patients may have been underestimated. Different studies recommend preserving the largest possible amount of thymus tissue during heart surgery in patients who are otherwise healthy, and particularly in the case of patients undergoing neonatal surgery.23,32,34 Our results support the mentioned recommendations and more so in the case of del22q11S patients, in who immune alterations are per se associated. We consider that more data of del22q11S patients infectious events need to be collected to further evaluate the consequences of this surgical procedure and should include long-term follow up, and measurement of lymphocyte function before and after surgery in this group of patients.
ConclusionPatients with del22q11.2S who are subject to partial or total thymus resection present lower lymphocyte and TREC indexes compared to those who have not undergone surgery. This situation may be influenced mainly by the age at the time of surgery and the amount of time elapsed since said surgery. We found no differences regarding the reported number of hospitalizations or infectious processes over the previous year between both studied groups with or without thymectomy.
Ethical disclosuresConfidentiality of dataThe authors declare that they have followed institutional protocols in regard to publication of patient data.
Right to privacy and informed consentThe authors obtained informed consent of patients and/or subjects referred to in the article consent. The corresponding author maintains responsibility for this manuscript.
Protection of human subjects and animals in researchThe authors declare that all procedures were carried out according to the ethical standards of the responsible committee on human experimentation and in accordance with World Medical Association and Declaration of Helsinki.
FundingThis research received federal funds (HIM 2012/023)
Conflict of interestThe authors have no conflict of interest to declare.
The authors thank Dr Normand García Hernández, from the Laboratory of Functional and Proteomic Genomics (IMSS), for the donation of material to measure TRECs.