INTRODUCTION
The great advances in science have provided insight to aspects in immunity that were practically not even suspected only a few years ago. The evolution of knowledge is so fast that the developments prove difficult to assimilate, and are soon forgotten - replaced by new findings.
The term immune modulation refers to changes in immune response, while immune suppression implies the suppression of such responses. Immune modulation in general is used to change immune responses that prove deleterious for humans. Such negative effects are usually the result of an excessive production of anomalous antibodies and sensitized cells that cause disease of an autoimmune or allergic nature. However, in some cases it is also of interest to modulate or change a normal response such as the rejection of a transplanted organ or tissue that the host immune system recognizes as foreign, in order to ensure tolerance of the transplant. It is also possible to modulate responses to improve immune reaction against infections, or as prophylaxis in children with immune defects such as either immaturity or primary immune deficiencies.
MONOCLONAL ANTIBODIES (MAbs)
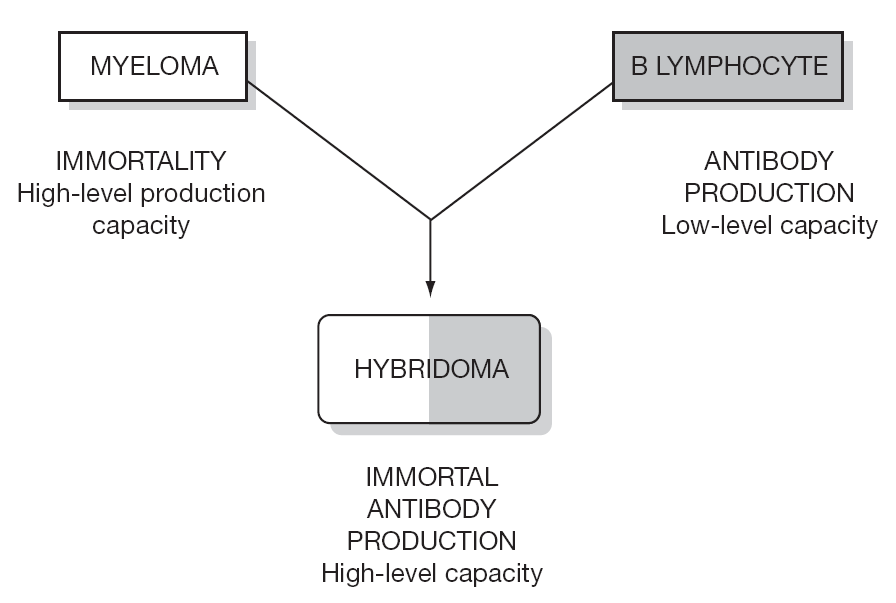
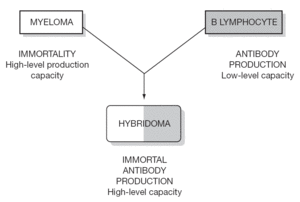
Monoclonal antibodies (MAbs) are proteins produced by hybrid cells (hybridomas) resulting from the fusion of a B lymphocyte (BL) capable of producing specific antibodies against a certain antigen, though in a limited manner, and a myeloma cell capable of unlimited production of nonspecific antibodies. The fusion between a BL and myeloma cell results in a mixture of cells from which a selection is made of only those cells that produce specific antibodies in an almost unlimited manner. These cells are called hybridomas, and produce MAbs that are all identical and are specifically targeted to a concrete antigen. The rest of the cells in the mixture are destroyed.
The laboratory production of these MAbs is simple. A rodent (rat, hamster, rabbit) is immunized with an antigen, after which B lymphocytes are extracted from the spleen or a lymph node. These cells produce specific antibodies against the antigen to which the rodent was immunized. These BLs are then fused with myeloma cells, followed by the selection of those fused cells or hybridomas that form MAbs on an unlimited basis, targeted against the antigen to which the rodent was immunized. The capacity to produce MAbs is enormous, and is only dependent upon the identification and isolation of the antigenic proteins, and immunization of the animal (rodent) with the latter (fig. 1).
Figure 1.--Schematic representation of MAb production.
The MAbs thus obtained are contaminated by DNA sequences of the rodent used to generate the sensitized BLs. As a result, on administering them to humans, they are recognized as foreign and trigger the production of human anti-mouse antibodies, with the induction of hypersensitivity phenomena and annulling the planned function of the MAbs. In order to avoid this problem, genetic engineering technology has been used to modify the MAb chains. The resulting part responsible for binding to the antigen (variable region) is minimal and of rodent origin, while the constant supporting part or region is of human origin. Consequently, tolerance of these modified MAbs is much greater. These modified molecules are known as humanized MAbs, since they contain 90 % of human material. These are the antibodies used in clinical practice, while non-humanized MAbs are used mainly in laboratories.
The capacity of a hybridoma to produce MAbs persists indefinitely, and they can be stored frozen and reconstituted at any time.
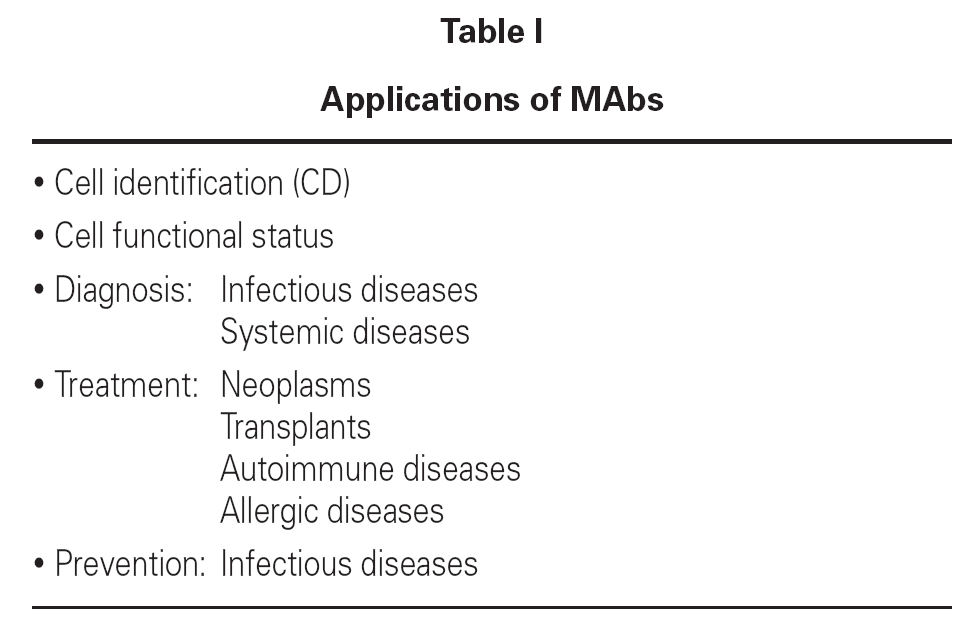
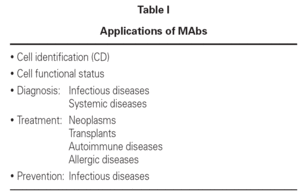
MAbs are used in a great variety of situations requiring sensitivity, high affinity and precision in relation to medical diagnoses and treatments, in veterinary practice, the food industry, in agriculture, etc.
In medicine, MAbs are used to identify cells by detecting cell surface antigens, identify human hematopoietic cell lines, and diagnose and treat viral or bacterial infections. They are also used to diagnose and treat tumors, prepare vaccines, modulate immune hyper-responsiveness, and ensure transplant tolerance. Further applications remain to de discovered1.
Some aspects of MAbs to be revised in this article refer to immune diagnosis, the diagnosis and treatment of neoplasms, the treatment of autoimmune diseases and allergic disorders, the prevention of respiratory syncytial virus (RSV) infection, and asthma in the nursing infant (table I).
MABS FOR DIAGNOSTIC PURPOSES
Identification of phenotypic markers
MAbs have been produced against all the cell surface markers and proteins, thus allowing the identification and classification of a broad range of cells, as well as the investigation of their functions. In the year 2000, a total of 247 cell antigens had been produced and officially acknowledged. The first MAbs against cell surface determinants were referred to with the letters "CD", followed by a number identifying basic cells of the immune system. Thus, anti-CD16 corresponds to a MAb that identifies natural killer cells (NK); anti-CD3 identifies T lymphocytes; anti-CD19-20 identifies BL; anti-CD4 identifies T helper cells; and anti-CD8 MAb identifies suppressor T cells. Binding of the specific MAb to the cell receptor is evidenced by immunofluorescent techniques, among other methods.
Diagnosis of neoplastic diseases
MAbs have been produced against surface antigens of tumor cells and molecules secreted by such cells, corresponding to a broad range of neoplasms. This has improved our knowledge of the biology of tumor cells, and has contributed to the development of new classification and diagnostic methods, the location of tumor cells, and the identification of specific tumor antigens that are exclusive of neoplastic cells. These tumor-associated antigens are phenotypical markers of cell function that have allowed the classification of leukemias and lymphomas.
MAbs USED IN THERAPY
The therapeutic application of MAbs is a developing field, though a number of promising results already have been obtained, such as the antibodies used in application to cancer treatment, allergic diseases and graft rejection in transplant patients.
Neoplasms
MAbs combined with antineoplastic drugs are specifically targeted to tumor cell receptors, thus avoiding damage to the healthy cells. MAbs also generate biological responses in the immune system; as a result, and in addition to direct cytotoxic action, they can induce antitumor responses via indirect mechanisms2.
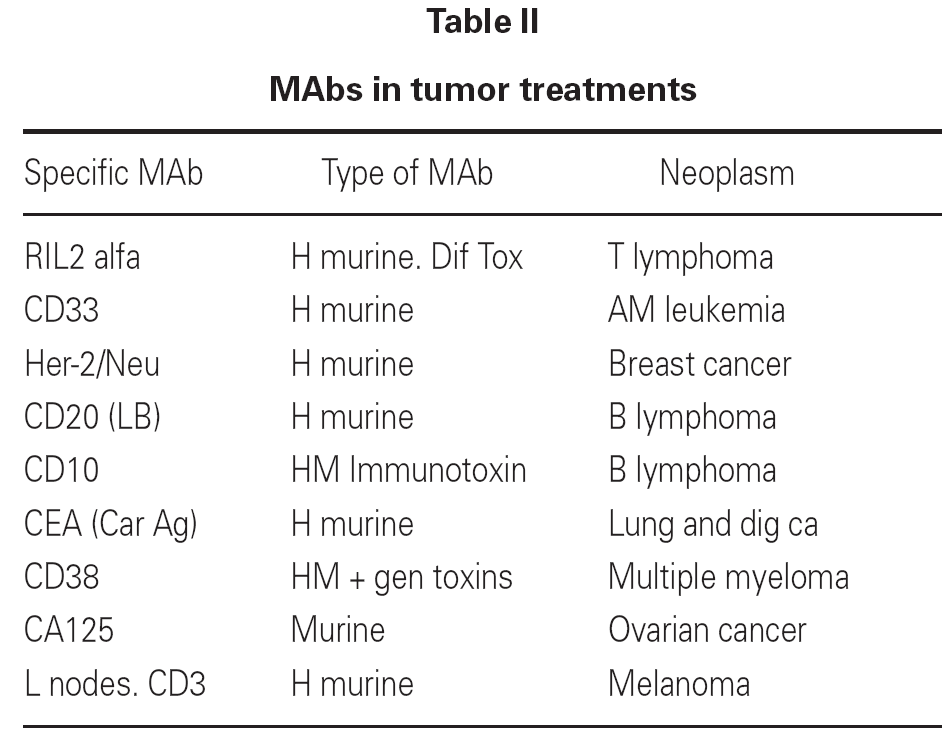
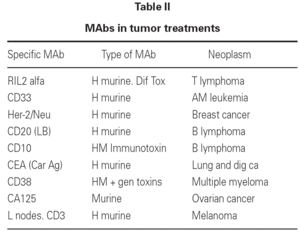
Examples of MAbs applied to neoplastic disease include the following: anti-CD20 (BL) (rituximab) is used to treat non-Hodgkin lymphomas and chronic lymphocytic leukemia. Anti-CD38 MAb is bound to a toxin in the treatment of multiple myeloma. Anti-R IL-2-alfa MAb, bound to diphtheria toxin at low doses, is used to treat T cell leukemias and T cell lymphomas. The binding of a MAb to a toxin that inhibits tumor protein synthesis yields a so-called immunotoxin3.
Anti-idiotype MAbs have been developed and used to treat B cell lymphomas that express surface immunoglobulins with concrete idiotypes.
Antitumor MAbs have also been applied to eliminate neoplastic cells from the bone marrow of cancer patients before autotransplantation. In this context, the bone marrow of the patient is extracted and treated with immunotoxins. Once cleared of neoplastic cells, the marrow is returned to the patient to reconstruct the hematopoietic system destroyed as a consequence of chemo- or radiotherapy. Table II shows some examples of MAbs used to treat cancer4.
Autoimmune diseases
Autoimmune diseases are produced as a consequence of anomalous T lymphocyte (TL) activation. In order to block such activation, MAbs can be used in different ways. Thus, anti-CD4 MAbs inhibit T helper cell function (T4), though it is also possible to block binding between CD40 and CD40 ligand (CD-40L). In this way, anti-CD40-L MAbs prevent binding to CD40 and therefore also co-stimulation, which is greatly increased in autoimmune diseases and in graft rejection. Moreover, tumor necrosis factor (TNF) is released in the pathogenesis of autoimmune diseases, and anti-TNF MAbs are able to exert blocking action in this context.
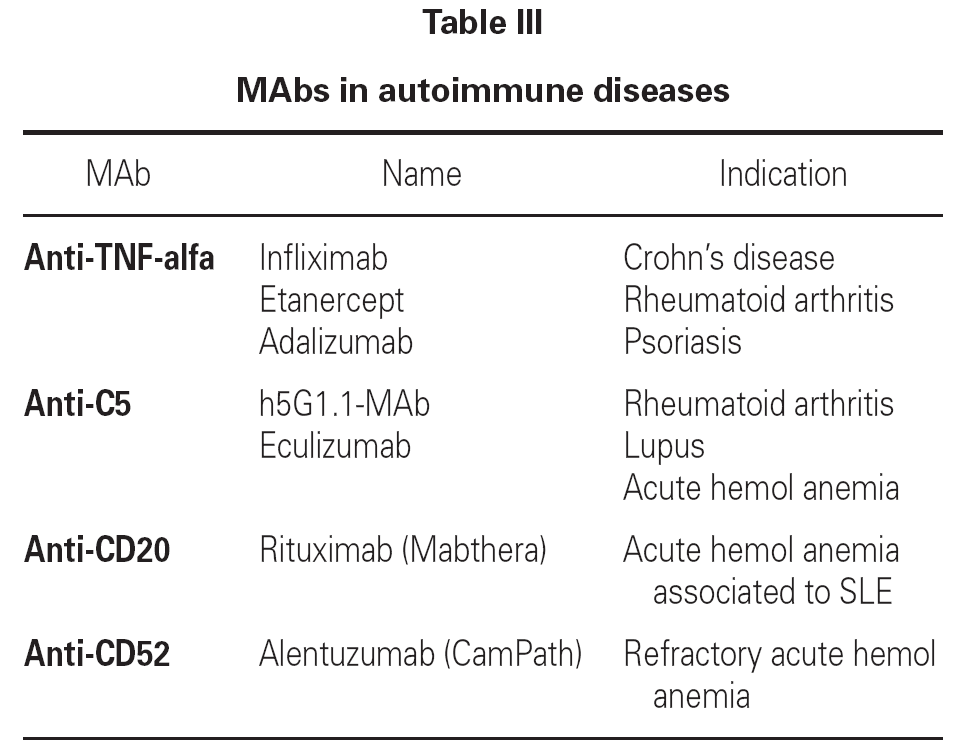
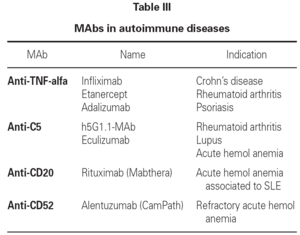
At present, the use of MAbs in application to autoimmune diseases has become quite widespread in both adults and in children, and constitutes a useful alternative in patients refractory to other forms of treatment. These MAbs are administered via the intravenous route on a hospital basis, and tolerance is generally excellent. Table III reports the MAbs used to treat autoimmune diseases, together with the precise indications.
This sense, experience has been gained in the treatment of acute anterior uveitis, idiomatic posterior uveitis, uveitis associated to Behçet's disease and chronic childhood arthritis refractory to other treatment modalities, using anti-TNF-alfa MAbs. The response is rapid and satisfactory in such cases5,6.
In refractory Crohn's disease, anti-TNF-alfa MAbs have been used with good results: improvement is observed in 65 % of the patients with doses of 5-10 mg/kg/week via the intravenous route, during four weeks7. In refractory chronic childhood arthritis, anti-TNF-alfa MAbs have obtained good results, associated to methotrexate. Tolerance is generally good - headaches and an increase in infections having been reported as side effects.
Treatment of graft rejection in transplant patients
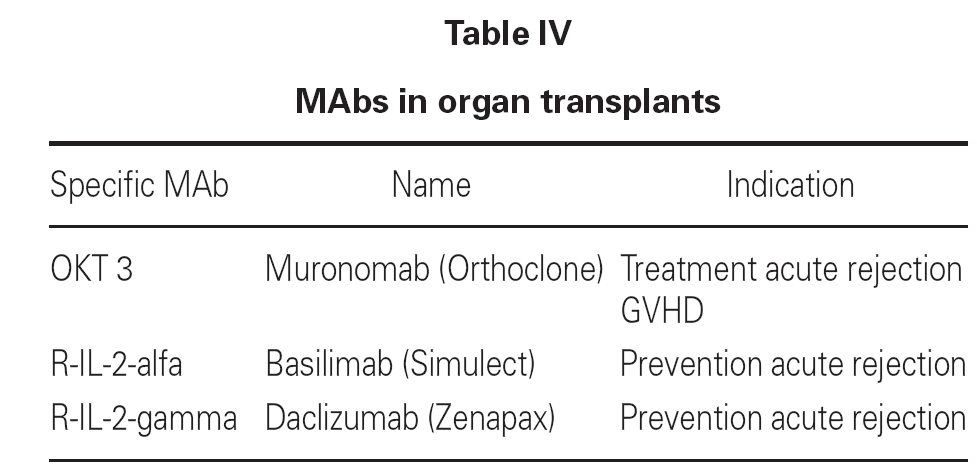
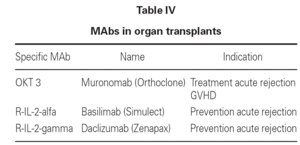
In the treatment of acute rejection in transplant patients, use is made of MAbs targeted to TL surface antigens, with the purpose of inhibiting their function and thus avoiding graft rejection. The most widely used antibody is OKT3 MAb, which specifically binds to TL surface antigen CD3. Complement activation and destruction of the T cell results upon binding.
Another MAb used in clinical practice is targeted to CD25, which is the alfa-subunit of the IL-2 receptor. In this context Il-2 binding to the T cells is blocked, and the latter are therefore not activated.
Both anti-CD3 and anti-CD25 are humanized murine MAbs that are widely used in clinical practice (table IV).
Allergic diseases
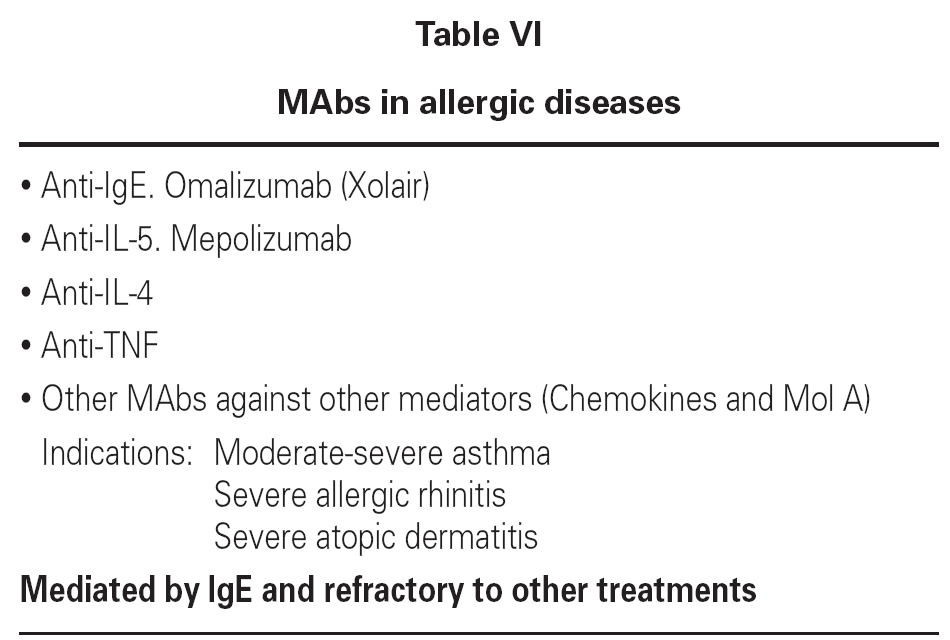
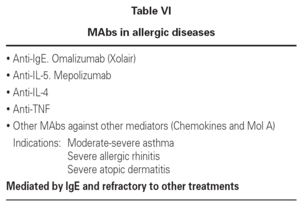
Allergic diseases produced as a result of type I hypersensitivity reactions and resistant to habitual treatment can be treated with humanized MAbs selectively targeted to the mediators of the allergic reaction: IgE, IL-5, IL-4, TNF-alfa, and others.
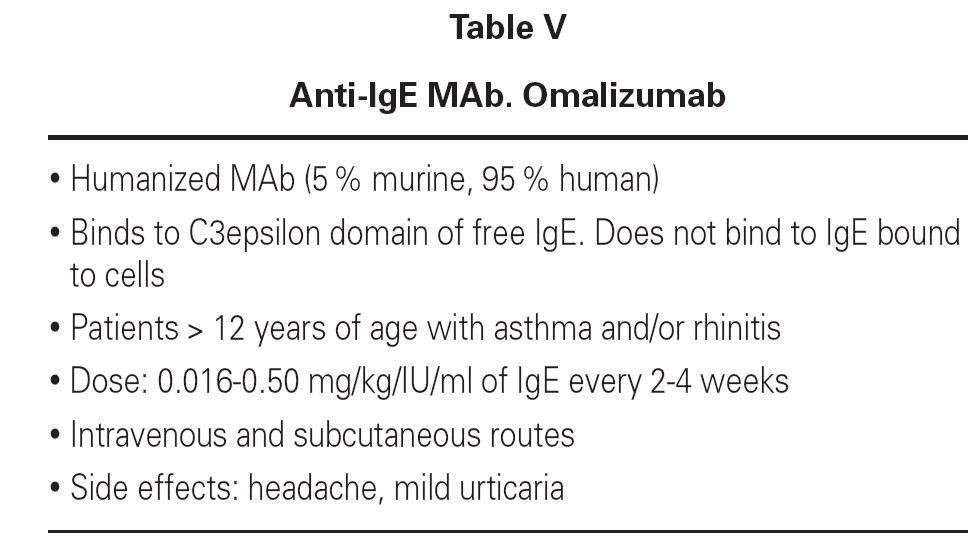
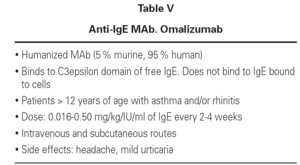
At present, humanized MAbs (5 % murine, 95 % human) targeted to IgE (omalizumab) are used in clinical practice with special indications and in patients over 12 years of age. The anti-IgE MAb rhu MAb-E25, known as omalizumab (Xolair®), selectively binds to the C3epsilon domain of free IgE, blocking binding to the high-affinity receptor. As a result, IgE does not bind to mast cells and basophils, and these cells therefore do not release their mediators. The MAb only binds to circulating IgE, not to other immunoglobulins, forming immune complexes that neither precipitate nor cause disease8-10. The drug can be administered intravenously or via the subcutaneous route at variable doses according to the levels of IgE in serum and the weight of the patient. The dosage ranges according to different authors between 0.016-0.50 mg/kg ever 2-4 weeks. These MAbs have been used in asthma and allergic rhinitis, producing a rapid decrease in serum IgE levels, in correlation with improvement of the clinical manifestations and patient quality of life. Tolerance is good - headaches and mild urticaria having been reported as side effects (table V)11,12.
In relation to humanized anti-IL-5 MAb (mepolizumab), it should be pointed out that IL-5 is essential for the recruitment, differentiation and maturation of eosinophils. These cells in turn play a key role in allergic reactions, including respiratory allergy and food and skin allergies. Anti-IL-5 MAb has been used in clinical trials in asthma, rhinitis, and atopic dermatitis. The result in terms of the reduction of peripheral blood eosinophil counts proved excellent, though this reduction was not correlated to clinical improvement of the patients13,14.
Other MAbs used to treat allergic diseases are indicated in table VI.
Prevention of bronchiolitis due to RSV infection and of asthma in the nursing infant
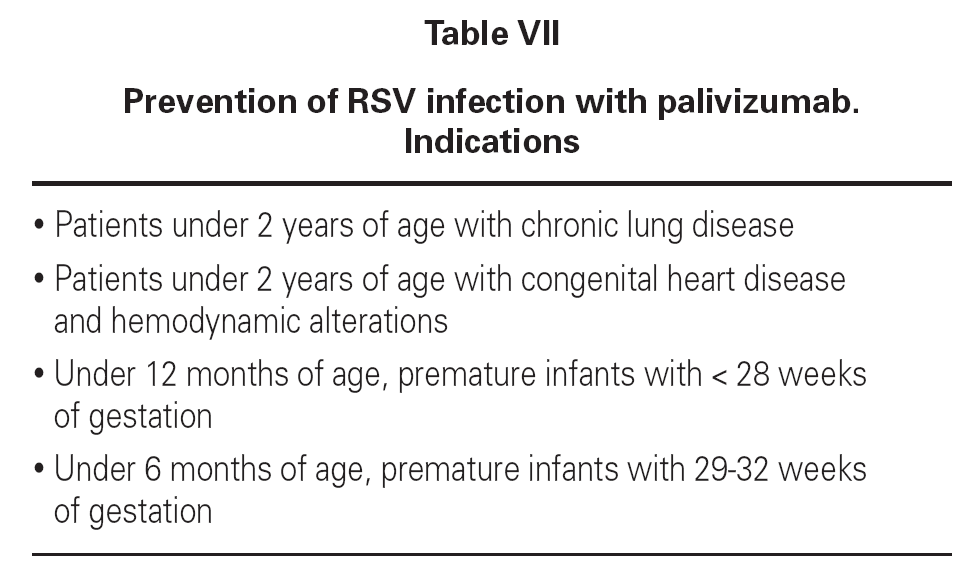
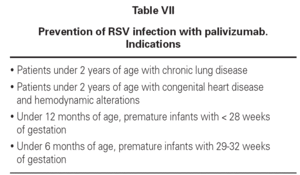
Newborn infants with chronic lung disease, congenital heart disease, infants with a gestational age of under 28 weeks, and premature infants with a gestational age of 29-35 weeks but with risk factors must be protected from respiratory syncytial virus primoinfection in the first year of life, due to their special immunity and clinical situation. Failure to prevent infection could lead to severe bronchiolitis and posterior dyspnea-producing bronchitis.
With strict clinical criteria and conducted on a hospital basis, treatment with class IgG humanized MAbs (palivizumab) specifically inhibits the antigenic A epitope of RSV protein F (fusion protein). This prevents the virus from fusing with and infecting the cells for posterior intracellular replication15.
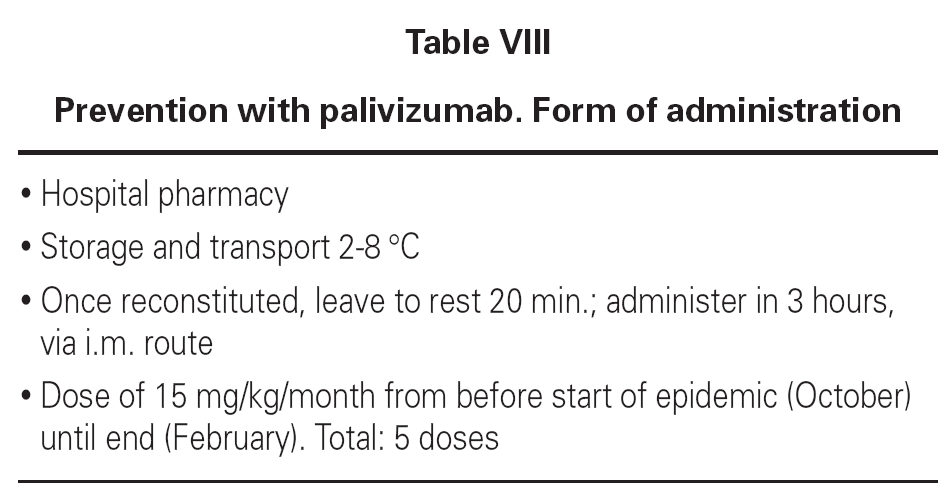
The drug is administered via the intramuscular route, though in premature infants with very low body weights the intravenous route can be used, at a dose of 15 mg/kg/month, in the months prior to the season in which the infection appears. A total of 4-5 doses are administered. This treatment does not interfere with the vaccination calendar. Tolerance and efficacy are excellent, avoiding the infection in most cases and reducing the recurrent wheezing episodes in the first year of life, as well as the number of hospital admissions (tables VII and VIII).
Non-inflammatory diseases
Anti-CD40 ligand (CD40-L) MAbs have been used in coronary disease, with partial results. In advanced coronary disease, associated to myocardial infarction, or unstable angina, anti-platelet aggregation factor MAbs (anti-GPIIb/IIIa MAbs) have been tested with good results in terms of the prevention of myocardial infarction16.
Correspondence:
Dra. M.A. Martin Mateos
Section Immunology-Allergy
Hospital Sant Joan de Déu
Pg. Sant Joan de Déu, 2
08950 Esplugues (Barcelona). Spain
E-mail: martinmateos@hsjdbcn.org