Mycoplasma pneumoniae is an intracellular bacterium leading to several complications in humans. M. pneumoniae is cleared in some cases and induces complications in others. The main responsible mechanisms regarding the controversy are yet to be cleared. Toll-like receptors (TLRs) are the important cell membrane and intracellular receptors which recognize a wide range of microbial macromolecules. The roles of TLRs in the eradication of several pathogens and also induction of their related complications have been demonstrated. This review article presents recent data about the roles of TLRs in the induction of immune responses which lead to M. pneumoniae eradication and related complications.
It has been reported that Mycoplasma are considered as the smallest cell-wall deficient prokaryote. The microbes are wall-less and malleable organisms which are able to grow and proliferate under cell-free conditions.1 Genetic investigations revealed that Mycoplasma contain a small genome (600–1350kbp) with 23–35% GC ratio. They belong to the bacterial class Mollicutes and nowadays seven species of Mycoplasma have been discovered which are pathogenic for humans, including Mycoplasma pneumoniae, M. genitalium, M. urealytium, M. fermentation, M. hominis, M. penetrans and M. pirum.2M. pneumoniae is the most evaluated species and is the most frequent cause of Mycoplasma-related human disorders.3 Epidemiological investigations demonstrated that although M. pneumoniae is distributed globally, its prevalence is local. Recent investigations demonstrated that the rate of M. pneumoniae infection is increasing annually.4M. pneumoniae is the main cause of primary atypical and community-acquired pneumonia.5 The diseases induce predominantly respiratory symptoms.5 The bacteria can also be considered as the cause of autoimmune hemolytic anemia and other blood-related diseases which lead to damage to the cardiovascular system and gastrointestinal tract. Thus, it participates in the pathogenesis of M. pneumoniae-related myocarditis, pericarditis, nephritis and also meningitis.6M. pneumoniae infection is complex because it is associated with several complications including cytoadherence, cytotoxicity, membrane fusion damage, invasive damage, toxic damage, nutrition depletion, and inflammation.6 However, the essential mechanisms used by M. pneumoniae to induce its complications remain to be elucidated.
It has been hypothesized that chronic recognition of M. pneumoniae by innate immune cells and consequently chronic activation of the cells may be considered as a main candidate to induce some of the important M. pneumoniae complications.7 Innate immune cells use several cytoplasmic membrane and intracytoplasmic receptors to recognize prokaryotes including M. pneumoniae.8 Toll-like receptors (TLRs) are the main well known receptors which play important roles in the recognition of prokaryotic macromolecules, entitled pathogen associated molecular patterns (PAMPs), and also endogenous damage associated molecular patterns (DAMPs).9,10 Thus, it has been hypothesized that the receptors may participate in the recognition of M. pneumoniae PAMPS to either the induction of appropriate immune responses against the microbe and clearance of the infection or the induction of chronic inflammation, which is a reason for M. pneumoniae complications.
Based on the introduction, this review article aims to present the roles of TLRs in the immune responses against M. pneumoniae and also their roles in the induction of M. pneumoniae complications.
Toll-like receptors introducingTLRs are the most important receptors that are expressed either on the cytoplasmic membrane (TLR1, TLR2, TLR4, TLR5 and TLR6) or in the intracytoplasmic vesicles (TLR3, TLR7, TLR8 and TLR9).10–12 The innate immune cell receptors have a similar structure containing three main domains including leucine rich repeat (LRR), intracytoplasmic and toll-IL-1 receptor domains.10–12 TLRs are different regarding LRR domain; so, they recognize various ranges of ligands that are microbial PAMPs. TLRs recognize their ligands in homodimeric and heterodimeric forms. TLR3, 4, 5, 7, 8 and 9 make homodimeric forms, while TLR1 and 6 make heterodimeric forms with TLR2.10–13 TLR2 is the only receptor which recognizes its ligands in both homodimeric and heterodimeric forms.14 The popular ligands for TLR 2 (in both forms), 3, 4, 5, 7, 8 and 9 are cell wall bacterial macromolecules, double strand RNA (dsRNA), lipopolysaccharide (LPS), bacterial flagellin, single strand RNA (ssRNA), ssRNA and prokaryotic DNA, respectively.15 TLRs use two popular distinct pathways, myeloid differentiation primary response gene 88 (MYD88) and TIR-domain-containing adapter-inducing interferon-β (TRIF) dependent pathways which are the responsible pathways to activate pro-inflammatory transcription factors including activator protein 1 (AP-1), nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), interferon regulatory factor (IRF) 3, IRF5 and IRF7.15 MYD88 dependent pathway are used by TLR2 (in both homodimeric and heterodimeric forms), TLR5, 7, 8 and 9, while TRIF dependent pathway is used by TLR3.16 TLR4 uses either MYD88 or TRIF dependent pathways.16 Activation of TLRs related transcription factors is associated with up-regulation of several molecules involved in the inflammation including co-stimulatory molecules and pro-inflammatory cytokines.16
Toll like receptors and Mycoplasma pneumoniae infectionBased on the introduction, all TLRs, except TLR5, can target M. pneumoniae because the bacterium contains the ligands for the innate immunity receptors. Interestingly, previous investigations have explored the roles played by TLR2 and TLR4 in the pathogenesis of M. pneumoniae only.
TLR2 and TLR4 play dual roles in M. pneumoniae infection, induction of appropriate immune responses to clear Mycoplasma pneumoniae and induction of M. pneumoniae complications (hyper-responsiveness).
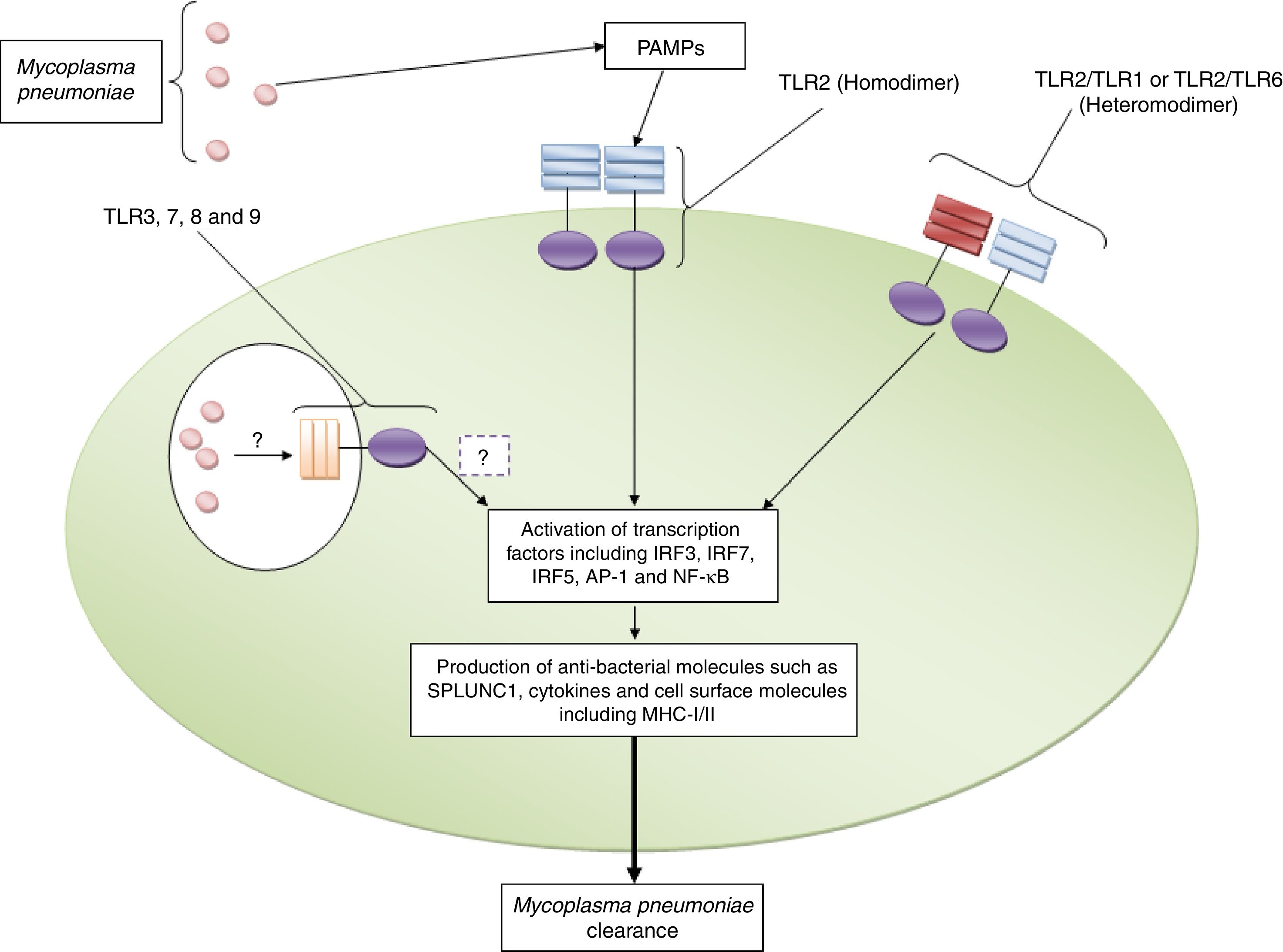
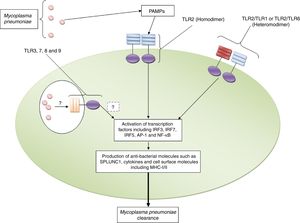
Roles played by toll like receptors in the induction of appropriate immune response against Mycoplasma pneumoniaeInvestigations have revealed that TLR2 induces immune responses against M. pneumoniae including up-regulation of pro-inflammatory cytokines and short palate lung and nasal epithelium clone 1 (SPLUNC1) which participate in fighting against the bacteria in the human lung.17 TLR2 dependent up-regulation of SPLUNC1 has also been reported by Chu et al.18 Another study demonstrated that TLR2 gene transfer to TLR2 knockout (KO) airway epithelium of M. pneumoniae infected BALB/c mice via adenoviral vector intranasal inoculation led to significantly reduced M. pneumoniae counts.19 The positive roles played by TLR2 in the clearance of M. pneumoniae in the established mouse allergic lungs have also been previously reported.20 Shimizu et al. reported that M. pneumoniae derived lipid-associated membrane protein activates TLR-2 homo/heterodimer leading to the activation of NF-κβ to elicit immune responses to M. pneumoniae.21 The crucial roles of MYD88, as an adaptor protein for TLRs, in the clearance of M. pneumoniae has also been documented by Lai et al.22Fig. 1 illustrates the roles played by TLR2 against M. pneumoniae.
TLR2 plays key roles in the recognition of Mycoplasma pneumoniae. The figure illustrates that TLR2 in both homodimeric and heterodimeric forms (with TLR1 and TLR6) recognizes Mycoplasma pneumoniae and induces appropriate immune responses to clear the bacterium. The roles played by TLR3, TLR7, TLR8 and TLR9 need to be explored.
Fan et al. reported that TLR2, but not TLR4, expression had positive correlations with severity of wheeze and IgE serum levels in the M. pneumoniae infected children.23 Another investigation by Shao et al. revealed that the expression of both TLR2 and TLR4 on the dendritic cells (DCs) had significant positive correlations with the severities of asthma in the M. pneumoniae infected patients.24 An investigation by Steib et al. demonstrated that TLRs activation on the nonparenchymal liver cells by M. pneumoniae is a main reason for induction of portal hypertension, especially in patients suffering from mal-liver functions such as liver cirrhosis.25 The positive association between expression levels of TLR4 and acute myocardial infarction in the M. pneumoniae infected patients have been reported by Lima-Neto et al.26 As mentioned previously, cytoadherence is a M. pneumoniae related complication which is associated with respiratory epithelium damage and immune response hypersensitivities.27 Accordingly, Shimizu et al. demonstrated that M. pneumoniae related cytoadherence is associated with inflammatory responses up-regulation of TLR4.28 However, the investigation was unable to find the roles of TLR2 in the induction of cytoadherence, as TLR2 KO mice reveal a normal cytoadherence complication like wild type (WT) mice.28 Another investigation revealed that M. pneumoniae cytopathic effects in the respiratory tracts may be associated with the interaction of M. pneumoniae lysate (MPL) with TLR2.29 Accordingly, Lee et al. used M. pneumoniae lysate to activate human airway epithelial cells to produce pro-inflammatory molecules such as cytokines.29 Their results demonstrated that M. pneumoniae lysate leads to up-regulation of pro-inflammatory molecules by human airway epithelial cells and using anti-TLR2 antibody and siRNA TLR2 leads to decrease expression of IL-8 as a pro-inflammatory cytokines.29 Nevertheless, Chmura et al. were unable to find the relation between expression of TLR2 and induction of IL-8 by M. pneumoniae.30 The important roles played by TLR2 in the induction of reactive oxygen species (ROS) and IL-8 by pulmonary epithelial cells have been documented by Choi et al.31Another study showed that TLR2 also induces M. pneumoniae related complications via up-regulation of prostaglandins PGD2 and PGE2 and also harmful pro-inflammatory cytokines such as TNF-α in a human macrophage cell line, RAW264.7 cells.32 Additionally, it has been documented that excess expression of mucins in the lung is the main complication of asthma.33 Kraft et al. demonstrated that M. pneumoniae induces expression of MUC5AC (the major airway mucin) via interaction with TLR2 in a pathologic manner.33 Chu et al. also confirmed the critical roles played by TLR2 in the up-regulation of mucin in the lung of M. pneumoniae infected patients.34 Nevertheless, the negative roles of TLR2 on the expression of mucin in the M. pneumoniae infected asthmatic patients have also reported previously.35 Interestingly, a study by Shimizu et al. revealed that M. pneumoniae derived triacylated lipoproteins activate NF-κB to induce excessive immune responses via interaction with TLR2 homodimer and also TLR1 and 2 heterodimer, but not TLR2 and 6 heterodimer.36 Another investigation by Chu et al. showed that inhaled fluticasone propionate (FP), a member of corticosteroids, leads to decreased inflammation and bronchial hyper-responsiveness via down-regulation of TLR2 in the lung.37 Furthermore, the significant roles played by TLR2 in the induction of cardiac and hepatic damages in the M. pneumoniae infected patients have been documented by Fan et al.38
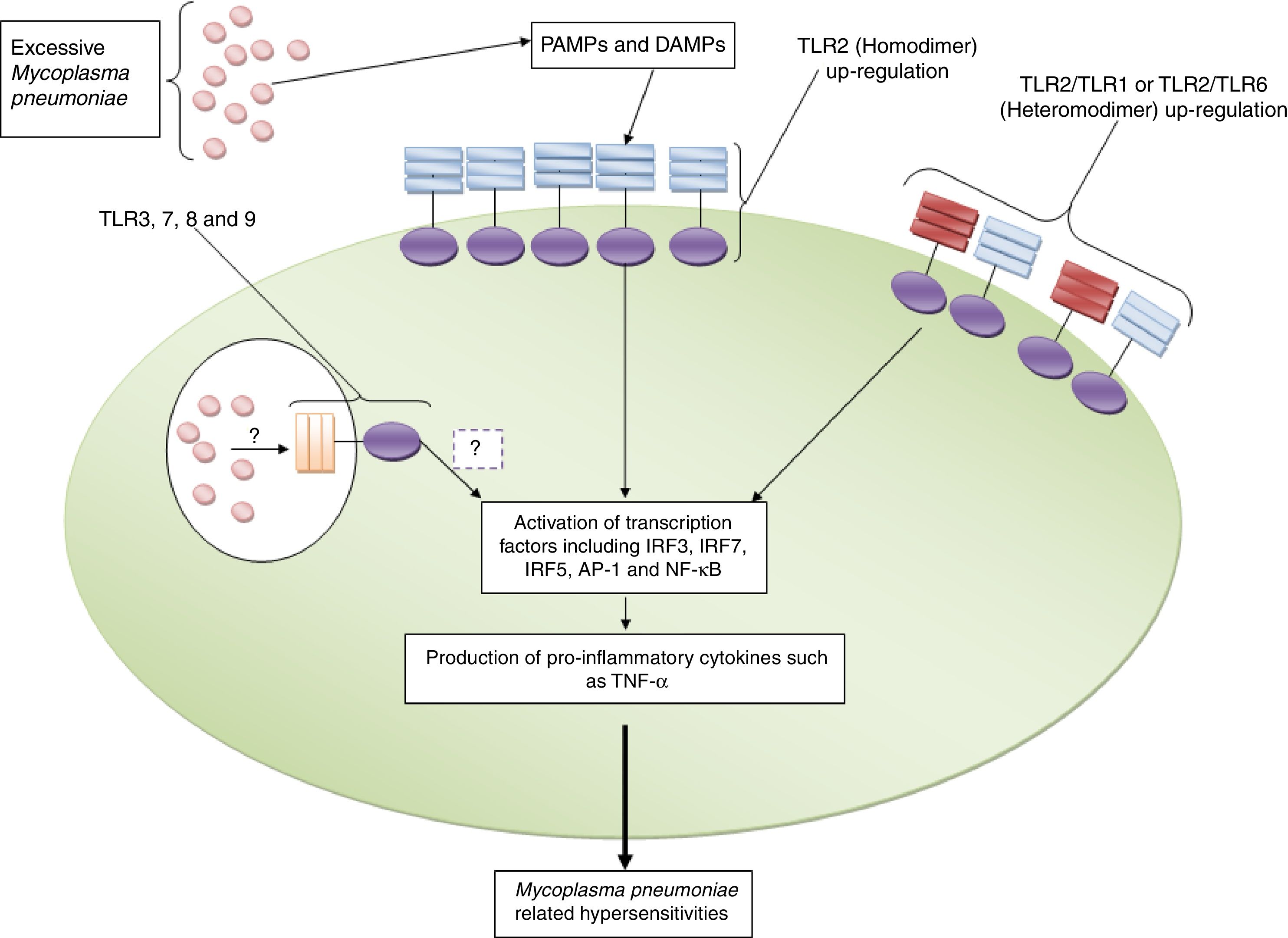
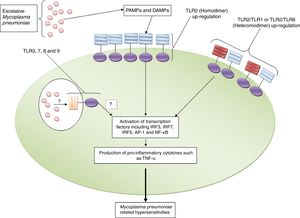
ConclusionAs mentioned previously, TLR2 and TLR4 are the only members that have been investigated regarding their roles in the induction of immune responses against M. pneumoniae and also their roles in the pathogenesis of the bacterium. Based on the fact that M. pneumoniae is a cell wall free microbe and its lipoproteins, which are anchored in the membrane, are exposed to immune cells, hence, it has been hypothesized that TLR2 is the most important TLR which participates in recognition of M. pneumoniae (Fig. 1). Accordingly, the investigations have revealed that TLR2 not only plays key roles in M. pneumoniae recognition; it plays significantly in the induction of M. pneumoniae related complications, especially hypersensitivities. It appears that expression levels of TLR2 and also counts of M. pneumoniae which are exposed to immune cells are the most important reason to determine the outcome of immune responses. Accordingly, excessive expression of TLR2 and its interaction with high numbers of M. pneumoniae results in hyper activation of immune cells and consequently induction of M. pneumoniae related complications (Fig. 2). Additionally, a study by Gally et al. showed that M. pneumoniae induces expression of TLR2 in a heat shock protein-1 (HSP-1) dependent manner.39 Therefore, it may be hypothesized that induction of endogenous DAMPs, such as HSPs, may be a mechanism used to induce hypersensitivities. Moreover, there is another hypothesis regarding the affinity of TLR2 to M. pneumoniae lipoproteins. Accordingly, it may be hypothesized that there is a magnitude affinity of TLR2 to M. pneumoniae diacylated lipopeptides which leads to induction of hypersensitivities.40
TLR2 and its roles in the induction of Mycoplasma pneumoniae related complications. The figure illustrates that excessive expression of TLR2 in both homodimeric and heterodimeric forms (with TLR1 and TLR6) and also high counts of Mycoplasma pneumoniae induce pro-inflammatory complications directly and by up-regulation of DAMPs (such as HSP-1) indirectly. The roles played by TLR3, TLR7, TLR8 and TLR9 in the induction of Mycoplasma pneumoniae-related complications need to be explored.
As mentioned, excessive expression of TLR2 on the immune cells may be a reason for induction of M. pneumoniae related complications. Several mechanisms may induce excessive expression of TLR2 including genetic variations. There is not enough investigation regarding the relation between genetic variations of TLR2 and M. pneumoniae complications. Fang et al. reported that the polymorphisms within TLR2 and TLR4 genes of pigs were associated with expression of the molecules in the M. pneumoniae infected animal.41 While, investigation on humans by Lima-Neto et al. showed that TLR4 c.896A> G, TLR4 c.1196> T and TLR2 c.2258G> A were not associated with increased risks of acute myocardial infarction in M. pneumoniae infected patients.26 Thus, it seems that more genetic and epigenetic investigation needs to be performed to improve our knowledge regarding the reasons of excessive expression of TLR2 in the patients suffering from M. pneumoniae.
Furthermore, due to the fact that intracellular localization is an important mechanism used by M. pneumoniae to escape from immune responses,42 hence, the intra-vesicular TLRs, including TLR3, TLR7, TLR8 and TLR9, can participate in the recognition of M. pneumoniae PAMPs and consequently play important roles in either its eradication or complications (Figs. 1 and 2). Thus, future investigations can be directed to exploring the roles of other TLRs in the immune responses against M. pneumoniae.
Conflict of interestThe authors have no conflict of interest to declare.
This review article has been supported by Islamic Azad University, Kerman Branch.