Allergic rhinitis, as an allergic and nasal hypersensitivity disease, is associated with the inflammation of nasal mucosa. It appears that innate immune receptors are the important risk factors in the pathogenesis of the inflammatory disease. Toll-like receptors (TLRs) are the most important receptors of innate immunity; their crucial roles in the recognition of allergens and subsequently pathogenesis of allergic diseases have been evaluated recently. TLR3, 7 and 8 are the intracellular members of the innate immune receptors and recognize intracellular single and double strand RNAs. This review article collected the investigations regarding the roles of TLR3, 7 and 8 in the allergic rhinitis pathogenesis.
Innate immunity is a main full consideration target for involvement in human pro-inflammatory diseases including allergic diseases.1 Innate immunity contains several receptors which have common features regarding ligand recognition.2,3 Innate immune receptors recognize ligands with similar features entitled pathogen associated molecular patterns (PAMPs) and damage associated molecular patterns (DAMPs).4 Toll-like receptors (TLRs), as the most known innate immune receptors, express either on the cell membrane or in the cytoplasmic vesicles.5,6 These molecules recognize a wide range of PAMPs/DAMPs and subsequently can participate in the recognition of several pathogens/allergens and also may participate in the induction of the immune related diseases.7 TLR3, 7 and 8 are the most important receptors which recognize single and double strand RNAs and subsequently activate pro-inflammatory transcription factors through two distinct intracellular signaling pathways.6,8 Accordingly, it has been hypothesized that the TLRs may participate in human pro-inflammatory based diseases.
Allergic rhinitis is a pro-inflammatory based disorder which is categorized as an allergic disease.1 The disease is associated with nasal airways damages and is prevalent in both developed and developing countries.9 Based on the crucial roles of innate immunity responses in human pro-inflammatory based diseases, it may be hypothesized that TLR3, 7 and 8 may play significant roles in the induction and progression of allergic rhinitis. This review article has aimed to describe the roles of TLR3, 7 and 8 in the pathogenesis of allergic rhinitis.
Description of allergic rhinitisAllergic rhinitis is associated with an inflammation in the nasal membrane10 in which eyes, ears, sinuses and oropharynx may be involved.9 After exposure to a specific allergen, IgE will be produced and binds to mast cells to produce sensitive mast cells which respond to re-entered allergens and accordingly some mediators will be released from mast cells.11 These mediators like histamine, tryptase, chymase, kinins, and heparin can lead to the symptoms like rhinorrhea, sneezing, itching, nasal congestion, redness, tearing, swelling, ear pressure and post nasal drip.12 Approximately 30–60 million people (10–30 present of adults and 40 present of children) are affected by allergic rhinitis in each year.13 However, mortality is rare but some morbidities like otitis media, eustachian tube dysfunction, sinusitis, nasal polyps, atopic dermatitis and allergic conjunctivitis are associated with allergic rhinitis.14 Treatment of allergic rhinitis according to treatment of allergic rhinitis by American family physicians is dependent on the severity of symptoms. The first line is second-generation oral or intra nasal antihistamine. Decongestants or nasal irrigation for nasal congestion, intranasal antihistamines or ipratropium for rhinorrhea, corticosteroids for mild to moderate symptoms intranasal and intranasal cromolyn and corticosteroids can be administrated in severe cases.15,16 Oral leukotriene receptor antagonist with oral or nasal antihistamine is considered and if the symptoms persist we can choose immunotherapy.17 Based on the effects of the anti-inflammatory effects of the chemotherapies on the expression and functions of TLRs, it has been hypothesized that TLRs may participate in the pathophysiology of allergic rhinitis. Additionally, based on the fact that the most current therapies can be associated with various side effects, thus, novel molecular therapies need to be explored for the treatment of this disease.17 Therefore, if TLR3, TLR7 and TLR8 participate in the pathophysiology of allergic rhinitis, hence, they may be considered as future targets for molecular therapies.
Introducing of TLR3, TLR7 and TLR8TLRs have a similar structure including, three well known domains, leucine-rich repeats (LRRs), transmembrane and Toll/interleukin-1 receptor (TIR). The LRRs domain is the section responsible for recognition of appropriate ligands, while TIR domain participate in activation of intracellular signaling pathways. TLRs recognize their ligand in both homodimeric and heterodimeric forms. TLR3, 7 and 8 recognize their ligand in homodimeric forms only in the intracellular cytoplasmic vesicles. TLR3 recognizes double-strand RNAs (dsRNAs), while TLR7 and TLR8 target double-strand RNAs (dsRNAs). TLR3/ligand interaction leads to activation of intracellular signaling in TIR-domain-containing adapter-inducing interferon-β (TRIF) dependent pathway, while, TLR7 and TLR8/ligands interactions result in activation of the intracellular signaling in myeloid differentiation primary response (MYD88) dependent pathway.
TLR3, 7 and 8 and their roles in allergic rhinitisAlthough an in vitro study by Globinska et al. revealed that innate immune responses to TLR3, TLR7 and TLR8 agonists were similar in nasal epithelial cells obtained from both patients with allergic rhinitis and healthy controls,18 based on the important roles of TLR3, TLR7 and TLR8 in recognition of ds/ss-RNAs, it has been hypothesized that expression and functions of the intracellular receptors may be complicated during allergic rhinitis. Interestingly, previous and recent investigations demonstrated that associations of TLR3, TLR7 and TLR8 with allergic rhinitis are different.
Accordingly one study demonstrated that mesenchymal stem cells derived from the patients suffering from allergic rhinitis express TLR3 more than other TLRs such as TLR5 and TLR2.19 Although mesenchymal stem cells play key roles in regulation of immune responses in the human tissues,20,21 they play controversial roles during allergic rhinitis and induce a significant increased activation of allergen-challenged lymphocytes from allergic rhinitis subjects and consequently increased expressions of inflammatory cytokines, major histocompatibility complex (MHC)-II and ligands for co-stimulatory factors, CD86.22 Increased expression of TLR3 on the mesenchymal stem cells in patients suffering from allergic rhinitis19 may propose that TLR3 may participate in the different functions of the cells during allergic rhinitis. In other words, it appears that TLR3 is a key molecule to induce pro-inflammatory properties of mesenchymal stem cells during allergic rhinitis. In addition to the roles of TLR3 in the altered functions of mesenchymal stem cells during allergic rhinitis, some investigations demonstrated that the innate immunity receptor may also participate in the deterioration of allergic rhinitis symptoms via increased sensitivities to infectious diseases which may be associated with pathogenic inflammation. Accordingly, Brandelius et al., demonstrated that airway challenge with dsRNA, the TLR3 ligand, had different responses in airway epithelial cells of the patients with allergic rhinitis when exposed and non-exposed to allergen.23 They revealed that exposure to allergens leads to increased innate immunity to viral infections when the cells were affected by dsRNA.23 Tengroth et al. also reported that TLR3 by induction of immune responses against pathogens participate in the exacerbation of the inflammatory condition during allergic rhinitis.24 Another investigation demonstrated that sRNA/TLR3 interaction is strongly associated with the production and activation of thymic stromal lymphopoietin (TSLP), which plays key roles in switching immune responses to allergic inflammation.25 Accordingly, Fuchimoto et al., showed that Humulus lupulus L. (Hop) water extract, an anti-inflammatory component, inhibits TSLP dsRNA/TLR3 dependent release from human nasal epithelial cells.25 Up-regulation of TLR3 in the nasal mucosa of the patients suffering from allergic rhinitis, especially during the pollen season has been demonstrated by Fransson et al.26 Collectively, it appears that TLR3 significantly participates in the induction of Th1 inflammation during allergic rhinitis. More investigations regarding Th2 responses against TLR3 approved the hypothesis. For example, Contoli et al. reported that Th2 cytokines, including IL-4 and IL-13, downregulate the expression of TLR3, and consequently IRF3, in the bronchial epithelial cell of patients suffering from allergic rhinitis.27 Mansson et al. also confirmed the results and reported that IL-4 and IL-5 down-regulates the expression of TLR3 in the eosinophils.28 The same results have also been derived from using TLR3 agonists. Accordingly, pre-treatment of airway epithelial cells with dsRNA led to up-regulation of IL-8 and consequently infiltration of eosinophils to the nasal in allergic rhinitis.28
In contrast to TLR3, it has been reported that TLR7 and TLR8, which recognize ssRNA rather than dsRNA, significantly participate in the amelioration of allergic rhinitis symptoms. For instance, a clinical trial study on the Swedish population with allergic rhinitis revealed that using AZD8848 as TLR7 agonist resulted in up-regulation of Th1 cytokines/chemokines (CXCL10, TNF-α, IL-6, IFN-γ) and suppression of Th2 lymphocyte functions in a profile which results in an improvement of allergic rhinitis symptoms.29 Intranasal use of AZD8848 by Greiff et al. has also been associated with decreased allergic rhinitis symptoms and pro-inflammatory molecules such as tryptase and α2-macroglobulin.30 Tsitoura et al. also examined the effects of GSK2245035, as another TLR7 agonist, and reported that intranasal application of the agonist is associated with expression of Th1 cytokine and suppression of allergic rhinitis symptoms in a safety profile.31 Administration of resiquimod (R848), as agonist for both TLR7 and TLR8, in in vitro conditions were also associated with the up-regulation of anti-inflammatory cytokines by peripheral blood mononuclear cells (PBMCs) which were obtained from atopic and non-atopic allergic rhinitis patients.32 Therefore, it appears that TLR7 and TLR8, as receptors for ssRNA, positively participate in the regulation of allergic rhinitis symptoms and can be considered as future targets for immunotherapy of allergic rhinitis. The important roles played by TLR7 and TLR8 may be confirmed by genetic investigations which revealed the significant association between genetic variations in the TLR7 and TLR8 genes and allergic rhinitis. For example, a study on a Swedish population revealed that rare variants within TLR7 gene significantly increased in the patients suffering from allergic rhinitis.33 Another study by Nilson et al. showed that two single nucleotide polymorphisms (SNPs) within TLR7 (rs2269809, and rs5935438) and five SNPs within TLR8 (rs3788935, rs3761624, rs17256081, rs4830805 and rs1548731) were associated with susceptibility to allergic rhinitis.34
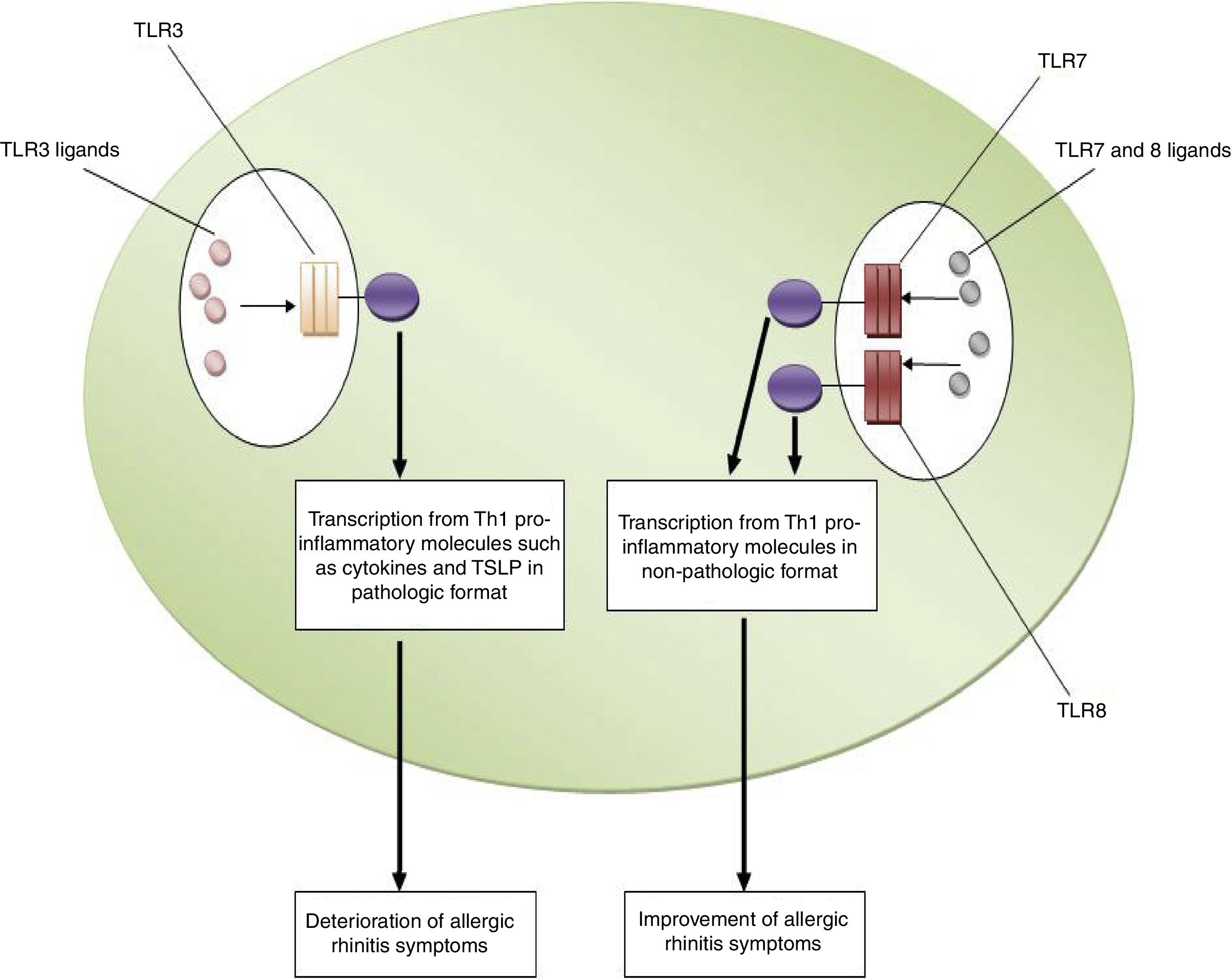
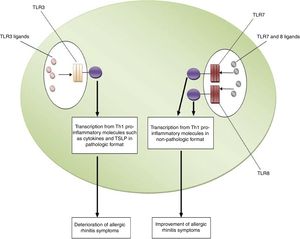
ConclusionsBased on the aforementioned data, increased expression of TLR3 and decreased expression of TLR7 and TLR8 may be considered as risk factors for the pathogenesis of allergic rhinitis. In other words, TLR3/ligands interactions significantly associated with the deterioration of allergic rhinitis symptoms via up-regulation of Th1 cytokine profiles in a pathologic format and also increased expression and activation of TSLP. However, interactions between TLR7 and TLR8/ligands are associated with the improvement of allergic rhinitis symptoms through the suppression of Th2 responses, the main causes of allergic rhinitis pathogenesis. Fig. 1 illustrates the main mechanisms used by TLR3, TLR7 and TLR8 in the pathogenesis of allergic rhinitis. Therefore, it may be hypothesized that using antagonists of TLR3 and also agonists of TLR7 and TLR8 may be considered for future immunotherapy of allergic rhinitis.
The roles played by TLR3, TLR7 and TLR8 in the pathogenesis of allergic rhinitis. The figure illustrates that interaction of TLR3 with its ligands, dsRNA, leads to up-regulation of Th1 related molecules in pathologic format which is associated with inflammation and subsequently deterioration of allergic rhinitis symptoms. However, interaction between TLR7/TLR8 and their ligands results in up-regulation of Th1 related molecules in non-pathologic format which is a reason for suppression of Th2 responses and subsequently improvement of allergic rhinitis symptoms.
None.
This project was supported by the Research committee of Kerman and Rafsanjan University of Medical Sciences.