Despite the major research investment in finding new efficacious and safe therapeutic options for severe uncontrolled asthmatic patients, omalizumab has been the only globally accepted recent innovative drug for allergic asthma. This recombinant DNA-derived humanised monoclonal antibody binds to IgE, ultimately decreasing allergic inflammation. Such therapeutic intervention, approved for use in Europe since 2005, had been focussed mainly on adults, notwithstanding the importance of allergy and the high prevalence of paediatric asthma. In fact, asthma is one of the most common chronic diseases in children, associated with considerable morbidity, particularly if the asthma is severe. It is the leading cause of hospital admissions in children with chronic disease, compromising both children and caregivers’ quality of life.1 These data provided a strong rationale for an investigation plan concerning omalizumab use in paediatrics, which led, in 2009, to the extension of this drug's approval to children aged 6–11 years old.2 For this, clinical trials regarding children were important, showing omalizumab to be both efficacious (stressing a reduced asthma exacerbation rate) and safe in this age group.3,4 Further randomised, double blind, placebo-controlled clinical studies have recently been published, highlighting omalizumab's effect on paediatric asthma control improvement and also addressing its safety.5–7 The next step is now to present and discuss real-life clinical data, which is the purpose of this study, concerning the recently fulfilled 52-weeks follow-up of our first omalizumab-treated patient under the age of 12. This anti-IgE therapy's effect on other allergic diseases besides asthma is also considered.
An 8-year-old boy with severe persistent uncontrolled allergic asthma since early infancy (first severe episode at the age of eight months old) was considered for omalizumab treatment. The child has concomitant moderate-severe persistent allergic rhinitis, mild-moderate atopic eczema and IgE-mediated food allergy (egg allergy until 7 years old and current peanut allergy with anaphylaxis), as well as asthma maternal history. Prior to the anti-IgE therapy, despite following an optimised asthma therapeutic plan including daily inhaled combination of long-acting β2-agonist (LABA) and high-dose inhaled corticosteroid (ICS), the child maintained nocturnal and diurnal wheezing, with impaired daily leisure and school activities. Compliance to therapy was closely assured by regular interview and physician-assessed correct inhalation technique as well as evaluation of medication use and prescriptions requirements. However, rescue medication had to be used often (near-daily inhaled salbutamol; oral corticosteroids at least twice a month) and the child had 12 hospital admissions due to asthma exacerbations. Systemic corticosteroids have been used for the shortest time needed, in order to avoid their unbearable side effects. These were regularly analysed, including growth rate and cortisol level evaluations, which have always been within the normal range.
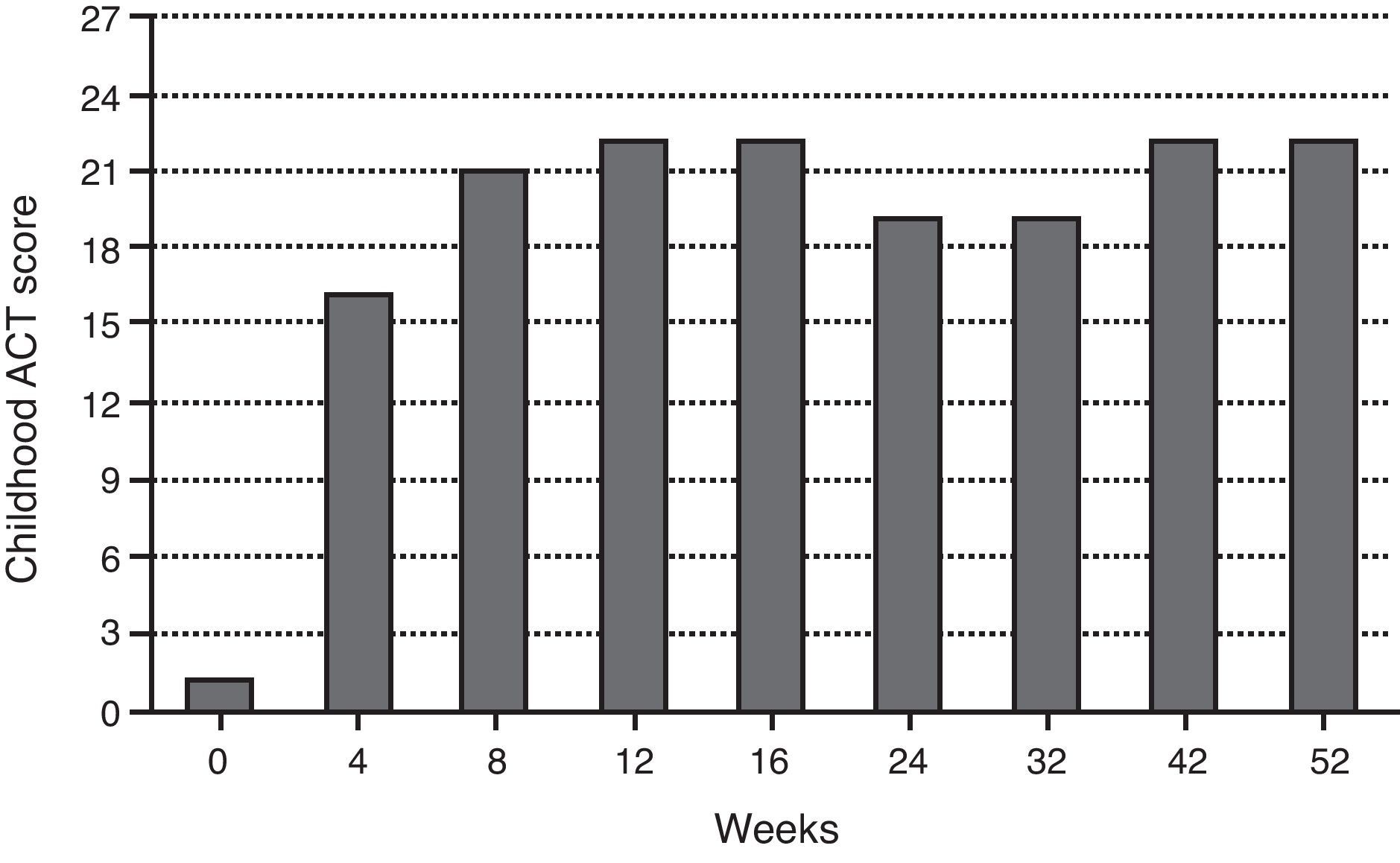
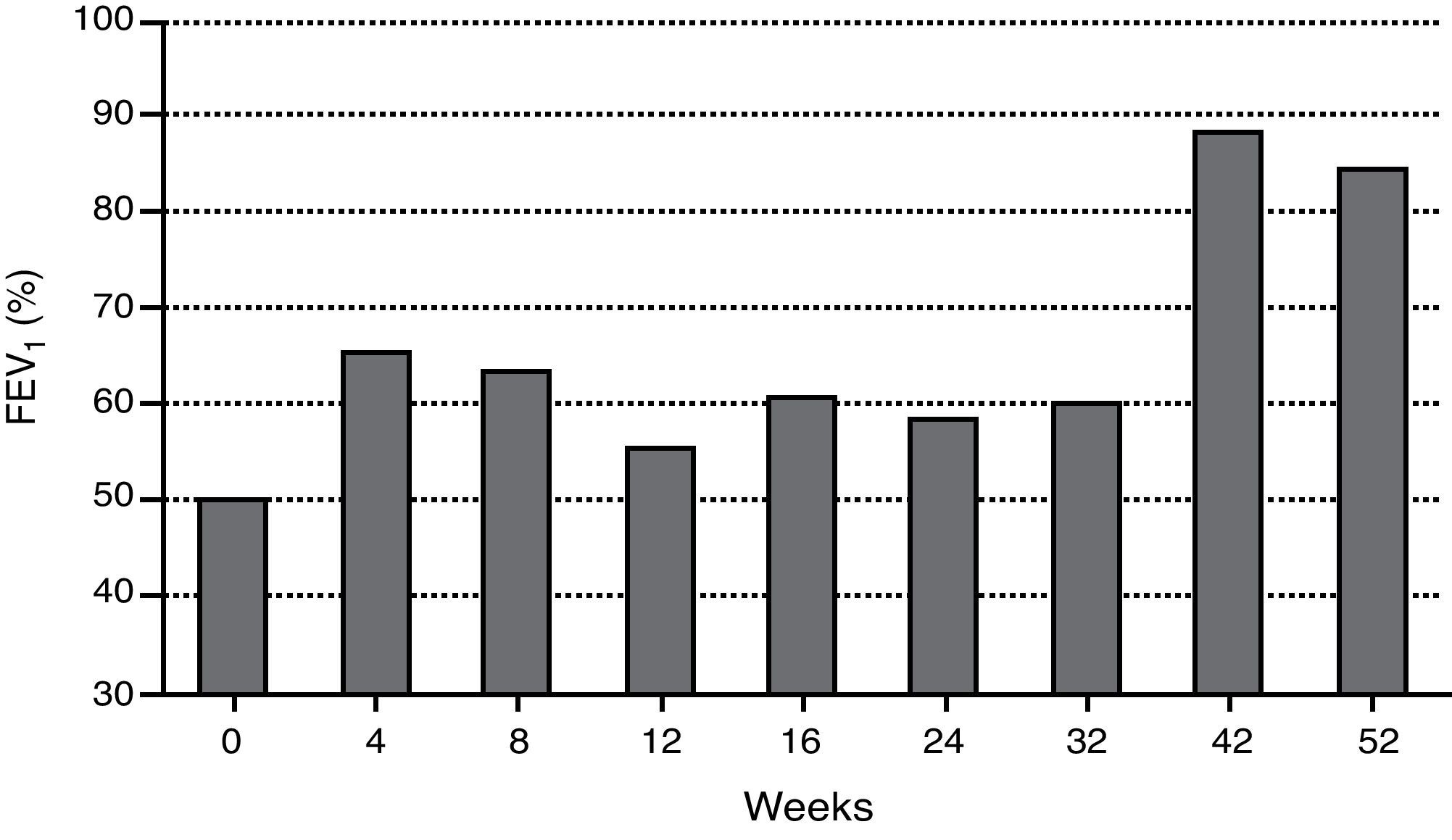
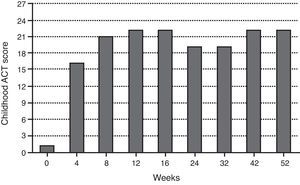
During the last year, the child had more than 12 severe asthma exacerbation episodes, requiring multiple unscheduled physician visits, leading to more than 30 days of school absenteeism. The Childhood Asthma Control Test (ACT) score before omalizumab initiation was one. Respiratory function tests showed severe obstructive ventilatory defect (forced expiratory volume in 1s (FEV1) of 49.7%), with positive bronchodilator response (post-bronchodilator increase in FEV1 of 21%). The fraction of exhaled nitric oxide (FeNO) was 45ppb. Skin prick tests (SPT) and specific IgE (sIgE) determinations were positive to common aeroallergens, namely mites (Dermatophagoides pteronyssinus, Dermatophagoides farinae, Lepidoglyphus destructor, Tyrophagus putrescentiae), grass pollen and cat epithelium, and negative to other aeroallergens, including moulds and cockroaches. The boy was also sensitised to peanut (sIgE 2.08kU/L). Eosinophilia (1070cells/μL) was noted on complete blood count. Other performed diagnostic exams were normal: SPT to Aspergillus fumigatus, computerised chest tomography, C-reactive protein, sedimentation rate, hepatic and renal function analysis, alpha-1-antitrypsin, sweat test and total immunoglobulins determination, except total serum IgE, which was elevated (915IU/mL). Smoke exposure was denied. Omalizumab additional treatment was decided according to weight (37kg) and total serum IgE: 375mg, subcutaneously administered, every two weeks. The patient's follow-up consisted of regular clinical evaluation every two weeks, comprising Childhood ACT questionnaire, asthma medication requirements, unscheduled physician visits due to exacerbations and lung function analysis, as well as rhinitis and eczema control (including SCORing Atopic Dermatitis – SCORAD evaluation).
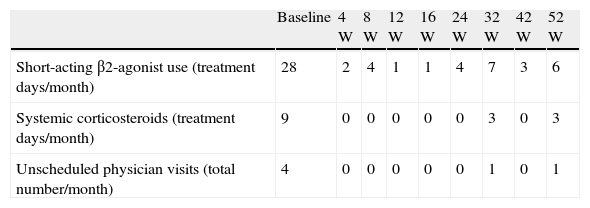
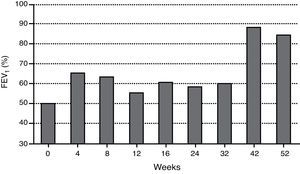
When anti-IgE was initiated, the patient was treated with high-dose daily-inhaled fluticasone (1500μg) plus salmeterol and ipratropium bromide via metered-dose inhaler use with a spacer (accurate inhaling technique) and oral montelukast and diprophylline. Since then, ipratropium bromide and diprophylline have been discontinued, ICS daily-dose has been reduced (1000μg) and no further hospital admissions have occurred. Table 1 shows a reduction in rescue medication use as well as unscheduled physician visits after omalizumab initiation. Two severe asthma exacerbation episodes occurred (weeks 28 and 47) in the context of an upper airway tract infection and probably associated with peak grass pollen exposure, respectively, which were easily controlled. Figs. 1 and 2 represent the improvement in asthma control, regarding symptoms and lung function, respectively. Normal FEV1 values were registered at 42 and 52 weeks of treatment (FEV1 at 52-week was 84%). The FeNO level at 52-week was 32ppb and peripheral blood eosinophilia persisted (760cells/μL).
Rescue medication use and number of unscheduled physician visits due to uncontrolled asthma at baseline and 4, 8, 12, 16, 24, 32, 42 and 52 weeks of treatment with omalizumab.
| Baseline | 4W | 8W | 12W | 16W | 24W | 32W | 42W | 52W | |
| Short-acting β2-agonist use (treatment days/month) | 28 | 2 | 4 | 1 | 1 | 4 | 7 | 3 | 6 |
| Systemic corticosteroids (treatment days/month) | 9 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 3 |
| Unscheduled physician visits (total number/month) | 4 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
W – week.
Note: baseline values relate to four weeks before omalizumab treatment initiation.
Allergic rhinitis has also improved, being controlled since the fourth week of treatment (except during the exacerbation due to peak grass pollen exposure), as opposed to eczema, which has shown no significant change (mean total SCORAD 18). The child maintained a peanut-free diet.
Pain at injections sites during omalizumab administration has been the only direct treatment adverse effect, which has been solved by the application of local anaesthetics (lidocaine and prilocaine, EMLA® patch) before the injections. Both child and parents subjectively evaluated the treatment favourably (ACT at 52-week was 22), highlighting his significant improvement in quality of life.
The concept of severe asthma is related to intrinsic characteristics of the disease, considering that associated and worsening factors have been excluded or treated and that there is compliance with therapy. Omalizumab is indicated as add-on therapy for children aged 6–12 years old with severe persistent allergic asthma, sensitised to a perennial aeroallergen and frequent daytime and night-time symptoms and who have had multiple documented severe asthma exacerbations despite daily high-dose ICS plus LABA.2 Therefore, all the criteria for omalizumab treatment were met in this case report. Additionally, this child also had decreased lung function, which is not a necessary criterion to decide for omalizumab therapy in this age group, as opposed to adults. This is mainly justified by the fact that most children with inadequately controlled asthma have normal or near-normal FEV1 values, in contrast to adult asthma.8
The continuous evaluation of efficacy and safety has supported omalizumab treatment maintenance in this case study since its early beginning, with significant effects observed after only four weeks, as has been also reported by Busse et al.7 Furthermore, these authors found the effect of omalizumab to be greatest in patients who were both sensitised and exposed to cockroach allergen and in those sensitised to dust mites, the latter being true in our case report. In fact, the patient's clinical condition has improved considerably, demonstrated by enhanced Childhood ACT scores (i.e., fewer asthma symptoms), reduction in rescue medication use, namely for systemic corticosteroids and fewer unscheduled physician visits due to asthma exacerbations (i.e., declined exacerbation rate and severity). Total ACT scores above 19 have been registered since the eighth week of omalizumab treatment. The improvement in asthma control also allowed a reduction in the daily-dose ICS, as stated in clinical trials.3,7
Lung function did not improve significantly during the first 32 weeks of omalizumab treatment. Inconsistent changes in children's lung function have also been stated in other studies.3,4,7 However, in this case study, normal FEV1 values were registered from week 42, despite a duration of almost 7 years of poorly controlled asthma, with associated severe, albeit variable, airflow obstruction. Besides long-term disease duration, the child's exposure to asthma exacerbating factors coinciding with early omalizumab treatment, namely respiratory viruses’ peak during autumn and winter seasons and grass pollen exposure in springtime may have contributed to the registered delayed lung function improvement.
The registered high FeNO levels underline persistent latent airway inflammation. Additionally, residual asthma symptoms complaints reflect that this patient's clinical condition, although unquestionably improved, was not yet completely controlled after 1 year of omalizumab treatment. Likewise, eosinophil counts showed only a slight decrease. Besides asthma, the other allergic co-morbidities may contribute to persistent high levels of inflammatory biomarkers.
Omalizumab might also be a new therapeutic opportunity for non-asthma conditions. However, sound evidence has only been provided regarding allergic rhinitis, showing omalizumab to be beneficial regarding symptom reduction,9 as has been registered in our patient. Its effect in eczema is still controversial, given different results.9 A reduction in IgE-mediated food allergy symptoms has been reported but further studies are needed.10
Pain due to drug injection may jeopardise omalizumab treatment tolerability, especially regarding younger children. This issue needs to be addressed, as the regular cutaneous application of local anaesthetics to children, though effective, is debatable.
In conclusion, the overall efficacy and safety analysis of this treatment in our patient has been highly positive, demonstrating omalizumab as an important additional therapeutic option for the control and consequent reduction in future risk in severe allergic asthmatic children. Long-term follow-up will further contribute to the benefit and risk assessment of omalizumab use in children.
Ethical disclosureProtection of human and animal subjects. The authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Confidentiality of data. The authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consent. The authors must have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence must be in possession of this document.