Antigen exposure and persistent inflammation leads to structural changes in the asthmatic airways which are collectively termed as “airway remodelling”. Presently available asthma medications ameliorate inflammations but are unable to prevent or reverse the airway remodelling process as most of the treatment strategies are only focused on inflammation instead of remodelling.
MethodsCurcumin, a phytochemical present in the rhizome of Curcuma longa is well known for its anti-inflammatory activity; however, the main drawback is its poor bioavailability which limits its therapeutic approval. So, the effect of nasal curcumin on acute and chronic asthma has been studied where short exposure to ovalbumin (4 days) represents acute phase whereas repeated exposures for longer (twice per week till 5 weeks) represents chronic asthma. Disodium cromoglycate (DSCG, 50mg/kg, i.p.) and dexamethasone (1mg/kg, i.p.) were used as standard drugs in acute and chronic model of asthma respectively.
ResultsOVA-induced airway inflammation initiated in acute stage led to remodelling due to persistent inflammation, epithelial and sub epithelial thickening (smooth muscle thickening), extracellular matrix (ECM) deposition, goblet cell hyperplasia and mucus plug formation. Intranasal curcumin is effective in inhibiting airway inflammation and remodelling both by maintaining the structural integrity of lungs in terms of inflammation, airway wall thickening and mucus production.
ConclusionOur findings suggest that curcumin administered through nasal route might prove therapeutically efficient in inhibiting allergic airway inflammations and maintaining structural integrity in the mouse model of allergic asthma. This may lead to the development of curcumin aerosol in near future.
Asthma is a complex, multifactorial and chronic inflammatory disease associated with bronchial inflammation and characterised pathologically by variable and recurring symptoms, reversible airflow obstruction, bronchospasm, airway hyperresponsiveness (AHR), airway inflammation and airway remodelling.1 Depending on the antigen exposure (dose and duration), it may be categorised as acute, sub-acute and chronic. Short-term exposure results in acute and sub-acute pathological condition and is considered to replicate airway inflammatory events whereas recurring, continuous and long term exposures result in chronic condition and structural alteration in the airways termed as “airway remodelling”, an important multi-cellular process refers to structural changes in the airway wall, which may include epithelial fragility, goblet cell metaplasia, increased airway smooth muscle mass and basement membrane thickening due to extracellular matrix deposition. Apart from airway inflammation, airway remodelling might be used as therapeutic target in the management of severe asthma. A variety of inflammatory cells such as eosinophils, T lymphocytes, mast cells, neutrophils, and dendritic cells (DCs), are recruited to the site of inflammation.
Eosinophils preferentially accumulate at sites of allergic inflammation and are believed to play important roles in the pathophysiology of asthma through the release of a variety of inflammatory mediators, including major basic protein (MBP), cysteinyl leukotrienes (CysLTs), radical oxygen species, and cytokines.2,3 In asthmatic patients with persistent sputum eosinophilia, treatment with anti-IL-5 mAb reduced asthma exacerbations and the requirement for systemic corticosteroids, and improved asthma-related quality of life (QOL). These results strongly suggest an essential role of eosinophils in the development of asthma exacerbations. Accumulating evidence suggests that eosinophils largely contribute to the development of airway remodelling.4–6 Both eosinophilic and neutrophilic inflammations may play an important role in severe asthma. It was suggested that severe asthma could be divided into two inflammatory subtypes: one, eosinophil (+) neutrophil (+) group, and the other is the eosinophil (−) neutrophil (+) group.7
Chronic inflammation is thought to have functional consequences that contribute to the structural changes in asthma which is recognised as the central component leading to remodelling.8 The structural changes in cellular phenotypes at the molecular level is primarily initiated by alterations in the airway epithelium, lamina propria and submucosa leading to the thickening of all the components of the airway wall (inner, outer, and total).9 Furthermore, continuous injury to the epithelium lead to its fragility in mild/acute asthma with extensive denudation, disruption, shedding and increased turnover of epithelial cells in severe/chronic asthma.10 Epithelial cell hypertrophy results in frequent differentiation of these cells to mucus-secreting goblet cells ensuing goblet cell hyperplasia/metaplasia and mucous gland hyperplasia. Apparent thickening of the epithelial basement due to increased deposition of interstitial collagen and other matrix proteins in the reticular basement membrane and peribronchial smooth muscle layer has also been reported which leads to subepithelial and peribronchiolar fibrosis (fibroblast hyperplasia).11 Similarly, altered matrix protein deposition contributes to remodelling of the sub mucosa and adventitia. Finally, the peribronchial area is thickened and there is evidence of disrupted or damaged alveolar attachments and abnormal elastin content in peripheral airways from severe asthmatics.12
Presently-used corticosteroids, theophylline and β2 agonists being the most potent anti-asthmatic drugs, are considerably successful in improving pulmonary airflow and inflammation both in acute and chronic asthma, but their effectiveness in reversing as well as controlling the structural remodelling events in the asthmatic airways has been limited.13,14 Since inflammation is considered as a driving force behind airway injury and repair, it has been proposed that asthma treatment might specifically and effectively target airway inflammation which would also affect the remodelling process.
Curcumin, a phytochemical derived from rhizomes of Curcuma longa has been considered safe in the treatment of a wide variety of disorders. Poor bioavailability limits its therapeutic applications. Recently, we have reported enhanced bioavailability of curcumin after intranasal administration increases its bioavailability in lung at lower dose. It has ameliorated histamine, eosinophil peroxidases and Th2 cytokine responses like IL-4, IL-5 in both acute and chronic asthma model without any side effect.15–16 We report here the initiation of structural changes like inflammation, smooth muscle thickening in lungs during acute phase which led to extracellular matrix (ECM) deposition, goblet cell hyperplasia and mucus plug formation after repeated OVA exposures for longer.
Materials and methodsAnimalsBALB/c mice (6–8 weeks old; 20–22g) were procured from Central Drug Research Institute (CDRI), Lucknow, India. They were maintained in a clean animal house under standard conditions of temperature (24±2°C), light/dark cycle and relative humidity (60–70%) in polypropylene cages, with rice husk as the bedding material. Experimental protocols of the current study were approved by the Central Animal Ethical Committee, Banaras Hindu University, Varanasi, India.
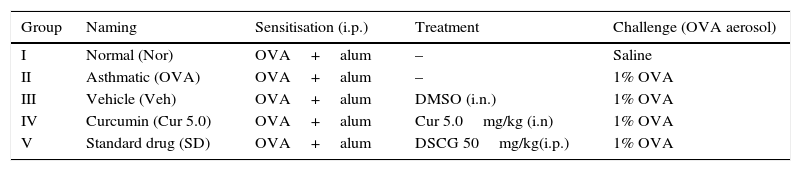
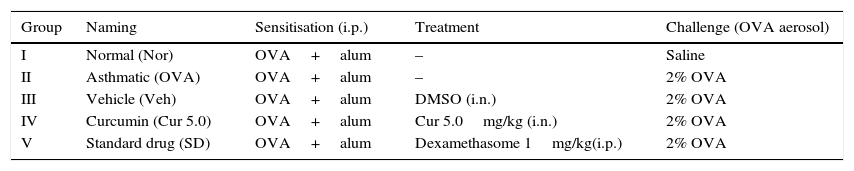
Grouping of animalsMice were randomly divided into two sets depending on the type of asthma: acute and chronic. For acute phase study, mice were divided into five groups (5 mice/group) and each group was named according to the status of sensitisation/challenge/treatment. Group I (normal), Group II (asthmatic), Group III (vehicle DMSO treated), Group IV (curcumin treated; 5.0mg/kg, i.n.) and Group V was DSCG (Disodium cromoglycate, 50mg/kg, i.p.) treated mice (Table 1).
Animal grouping for acute asthma.
| Group | Naming | Sensitisation (i.p.) | Treatment | Challenge (OVA aerosol) |
|---|---|---|---|---|
| I | Normal (Nor) | OVA+alum | – | Saline |
| II | Asthmatic (OVA) | OVA+alum | – | 1% OVA |
| III | Vehicle (Veh) | OVA+alum | DMSO (i.n.) | 1% OVA |
| IV | Curcumin (Cur 5.0) | OVA+alum | Cur 5.0mg/kg (i.n) | 1% OVA |
| V | Standard drug (SD) | OVA+alum | DSCG 50mg/kg(i.p.) | 1% OVA |
For chronic asthma model, animals were divided into five groups (5 mice/group) as in Table 2 and each group was named according to the status of sensitisation/challenge/treatment. Groups I to IV were similar as above except Group V which was dexamathasone (1mg/kg, i.p.) treated mice.
Animal grouping for chronic asthma.
| Group | Naming | Sensitisation (i.p.) | Treatment | Challenge (OVA aerosol) |
|---|---|---|---|---|
| I | Normal (Nor) | OVA+alum | – | Saline |
| II | Asthmatic (OVA) | OVA+alum | – | 2% OVA |
| III | Vehicle (Veh) | OVA+alum | DMSO (i.n.) | 2% OVA |
| IV | Curcumin (Cur 5.0) | OVA+alum | Cur 5.0mg/kg (i.n.) | 2% OVA |
| V | Standard drug (SD) | OVA+alum | Dexamethasome 1mg/kg(i.p.) | 2% OVA |
OVA: ovalbumin; DMSO: dimethyl sulphoxide; DSCG: disodium cromoglycate.
Balb/c mice (22–25g; 6–8 weeks) were randomly divided into two sets and each set was further divided into five groups (5 mice/group).
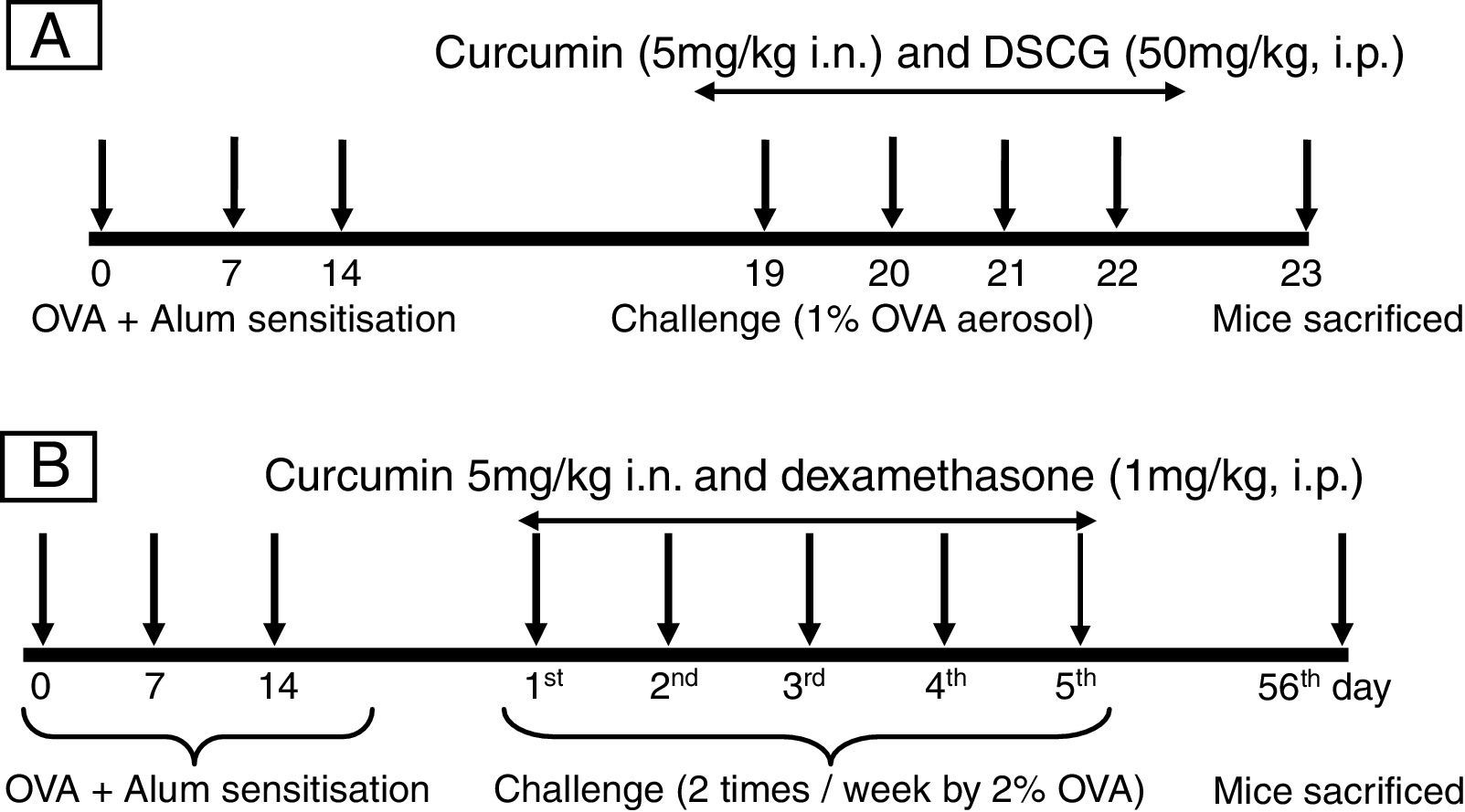
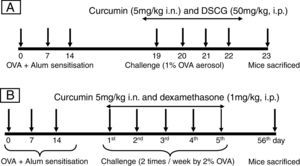
For developing acute allergic asthma model, mice were immunised with 0.2ml saline suspension containing 50μg Ovalbumin (OVA, Grade V: Sigma; St. Louis, MO, USA) emulsified in 4mg of aluminium hydroxide (Pierce; Rockford, IL, USA) on days 0, 7, and 14. From days 19 to 22, mice were subjected to aerosol of 1% OVA (prepared in saline w/v) inhalation 30min daily (Fig. 1A) in a Plexiglas chamber by nebuliser (Omran, Japan) with an airflow rate 9l/min. Control mice received i.p. injections of 0.2ml saline containing 4mg alum and were challenged with saline alone. Curcumin was dissolved in dimethyl sulphoxide (DMSO) and given an hour before every OVA challenge (days 19–22). Vehicle (DMSO) was also given to OVA-sensitised mice in a similar volume as curcumin. DSCG (di sodium cromoglycate) was used as a standard drug for acute model. Mice were sacrificed on day 24 (24h after the last challenge day).
Protocol for inducing experimental acute and chronic airway inflammation models. (A) In the acute model, mice were sensitised by three intraperitoneal injections of OVA on days 0, 7 and 14, and then received aerosolised OVA challenge on day 19–22 using a nebuliser. Mice were sacrificed on the 23rd day after 24h of last OVA challenge. (B) In the chronic model sensitisation was carried out in the same manner and mice were challenged by 2% OVA for the period of 5 weeks, twice in a week. Mice were sacrificed on the 56th day after 24h of last OVA challenge.
In the chronic asthma model, the sensitisation and challenge protocol was slightly modified.17,18 Briefly, all mice were sensitised with 50μg OVA adsorbed in 4mg aluminium hydroxide by intraperitoneal route on days 0, 7 and 14. From day 21 mice were subjected to 2% OVA aerosol for 30min, twice a week for the period of 5 weeks (Fig. 2B). Control mice received i.p. injections of 0.2ml saline containing 4mg alum and were challenged with saline alone. Curcumin was given an hour before every OVA challenge from day 21 to 55. Mice were sacrificed on day 56 (24h after the last challenge day). Dexamethasone was used as standard drug in the chronic model.
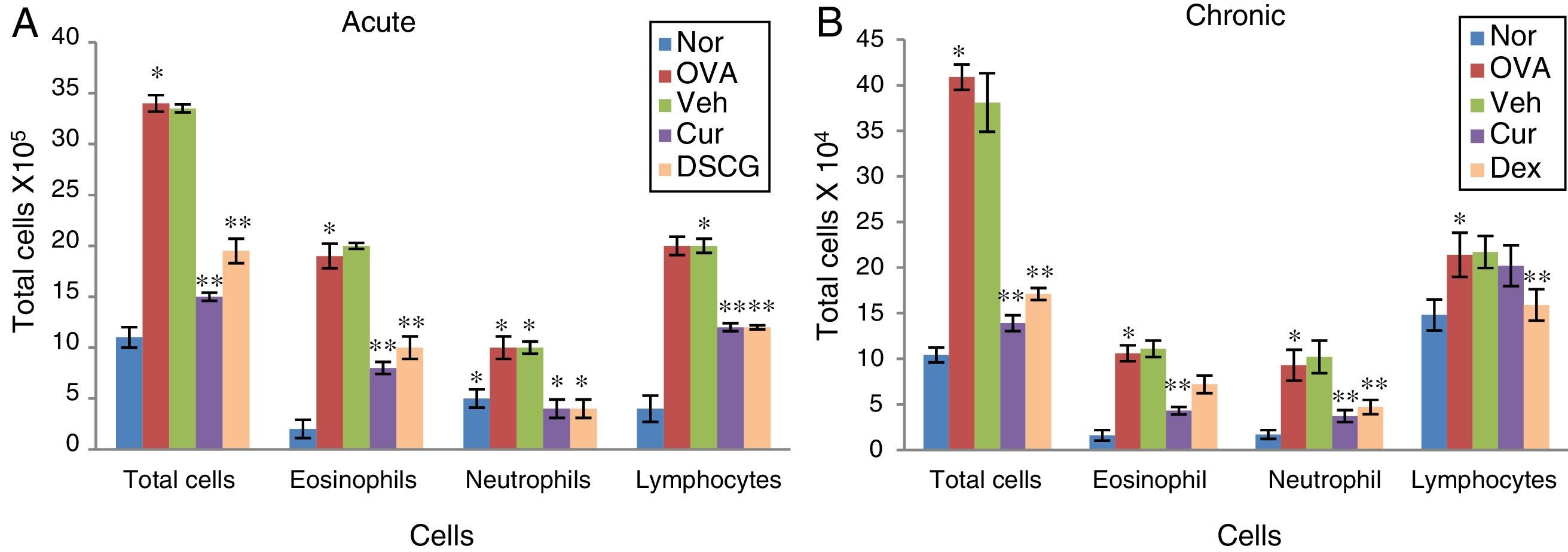
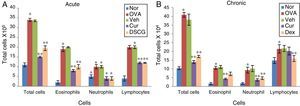
Effect of curcumin on inflammatory cell recruitment: BALF was centrifuged and the pellet was assessed for total cell count by Trypan blue dye exclusion test and differential count on cytospun slides by Giemsa staining in acute (A) and chronic (B) model. The cells were counted and identified as eosinophils, lymphocytes and neutrophils on the basis of their nuclear morphology. Total cell count encompasses increased eosinophils in OVA sensitised and challenged mice. Total cells and eosinophils count both were decreased with pre-treatment of curcumin in both acute and chronic model. The results are shown in means ±SEMs. *p<0.05 nor Vs OVA group and **p<0.05 OVA Vs cur group. Nor – normal; OVA – asthmatic; Veh – vehicle; SD – standard drug (DSCG in acute and dexamethasone in chronic).
After the last OVA aerosol, mice were sacrificed by cervical dislocation. BALF was collected by tracheal cannulation and obtained by washing of the airway lumen. Briefly, lungs were washed three times with 1ml of ice-cold PBS and were centrifuged at 3000rpm for 10min at 4°C. Cell pellet was used to analyse inflammation by total inflammatory cell count and differential cell count, especially eosinophils, neutrophils and lymphocytes. Lungs were inflated with 10% neutral buffered formalin and then removed aseptically and fixed in 10% neutral buffered formalin for 24h. After dehydration the lung tissue was embedded in paraffin, then cut into 5μm thickness sections, and processed further for staining with Haematoxylin–Eosin (H&E), Periodic Acid-Schiff (PAS), Masson's trichrome (MT) and Sirius red (SR). Dexamethasone group was not used for the histological grading analysis since 40–60% mortality was observed.
The degree of inflammation in airway was scored with special reference to the inflammatory cells recruited to the lungs in the peribronchiolar region.19 Lung sections were stained with Haematoxylin and eosin for general morphology and inflammation (inflammatory cell infiltration, epithelial layer thickening, sub-epithelial thickening/smooth muscle thickening). To determine the inflammatory cells infiltration in peribronchial region, cells were counted based on a 5-point scoring system as described by Myou et al.19 Briefly, the scoring system was: 0 – no cells; 1 – a few cells; 2 – a ring of cells 1 cell layer deep; 3 – a ring of cells 2–4 cells deep; 4 – a ring of cells >4 cells deep.
The thicknesses of epithelium and sub epithelial smooth muscle layers was also performed in H&E stained slides and measurements were taken from four points of each airway at 3, 6, 9, and 12 o’clock positions20 (Motic image plus software). Approximately 15–20 airways were evaluated for this study.
Masson's trichome staining was used for detection of extracellular matrix (Collagen) deposition in peribronchiolar region which was further confirmed by Sirius Red staining: 0–3 scoring system was applied to each observed in bronchi.21 Briefly, the scoring system was: 0 – no collagen deposition; 1 – a thin layer of collagen; 2 – a cluster of collagen; and 3 – a thick layer of collagen.
Lung sections were stained with PAS and counter stained with Haematoxylin to detect mucus containing goblet cells. Briefly, mucus content in the cell was scored 0–4 according to the criteria adopted by a grading system where 0 represents no goblet cells, 1<25%, 2<25–50%, 3<50–75% and 4>75%. All scoring systems were performed in at least 10–15 different fields. Mean scores were obtained in these fields.
Image capture and photomicrographsImages of lungs sections were captured with a digital camera (Motic). Briefly, a maximum of 10–15 bronchi, were analysed for all the parameters described. The measurement was performed using bar feature of Motic image plus software.
Statistical analysisExperiments were repeated thrice with consistent results and expressed as mean±SEM for the number of experiment with five animals/group. Statistical evaluation of the results to determine difference between all groups was performed using student t test. Results with p values less than 0.05 were considered significant.
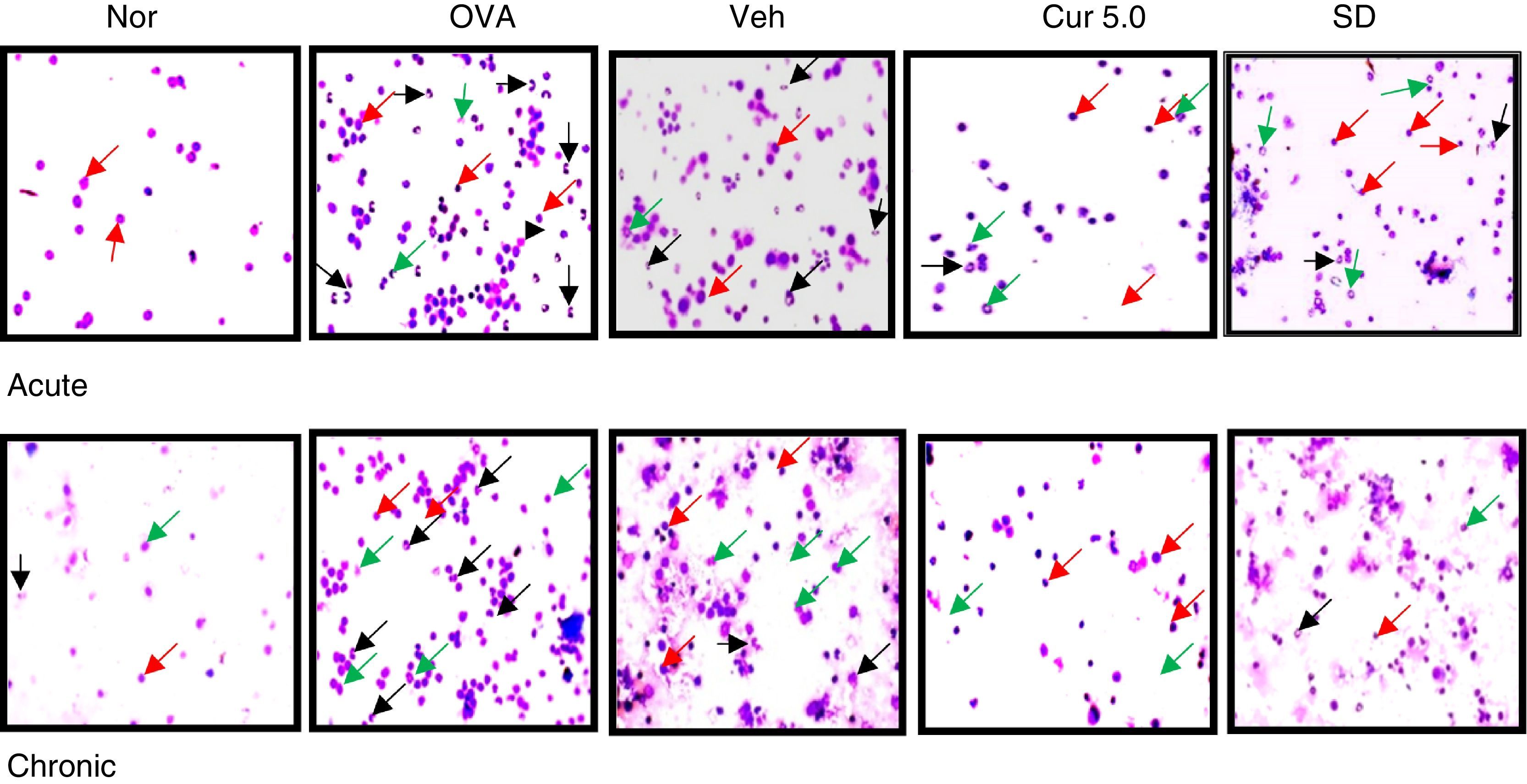
ResultsEffect of curcumin on total inflammatory and differential cell count in BALFThe total and differential inflammatory cell count was remarkably and significantly increased in both the asthmatic models, acute and chronic as compared to control mice which signifies the inflammation due to repeated OVA exposures (Fig. 2A&B). In acute asthma, a significant increase in total inflammatory cells was noted as compared to the chronic model. The increase in total number of cells was further associated with a significant increase in eosinophils, lymphocytes and neutrophils to some extent as observed in cytospun slides (Fig. 3A&B). In the BALF, eosinophils numbers were higher in acute asthma as compared to chronic model. Intranasal curcumin (5mg/kg) has significantly inhibited (more than 50%) the recruitment of inflammatory cells along with the eosinophils to the lungs in both acute and chronic models.
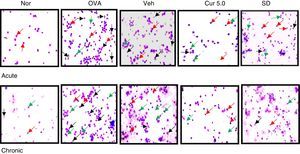
Differential cell count on cytospun slides 40×. The cytospun of the BALF stained with giemsa showed reduction in the recruitment of eosinophils after curcumin treatment in acute and chronic model. Apart from eosinophils; neutrophils and lymphocytes were also observed. Black arrow represent eosinophils; red arrow - lymphocytes while green arrow represent neutrophils. Nor – normal; OVA – asthmatic; Veh – vehicle; SD – standard drug used (DSCG in acute and dexamethasone in chronic).
Inflammatory responses to peribronchiolar region were assessed by histological evaluation of H&E-stained lung-tissue sections. To determine the inflammatory cells infiltration in the peribronchial region, cells were counted based on a 5-point grading system, where 0 – no cells; 1 – a few cells; 2 – a ring of cells 1 cell layer deep; 3 – a ring of cells 2–4 cells deep; 4 – a ring of cells >4 cells deep.
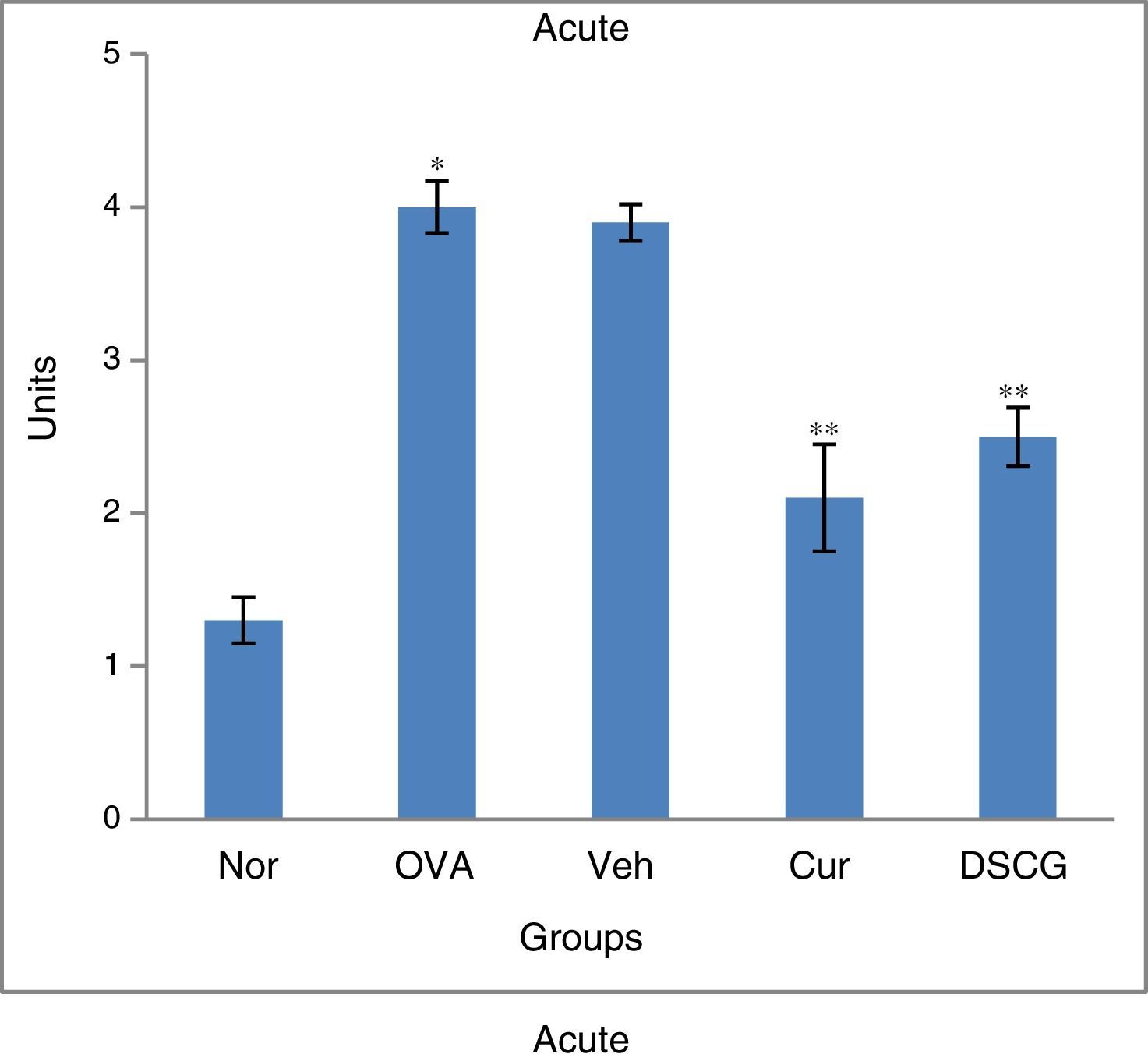
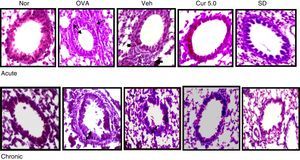
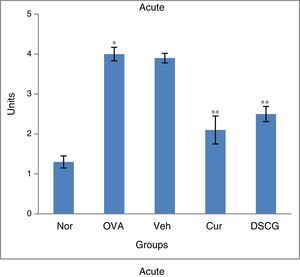
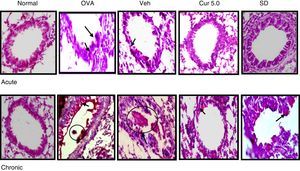
Negligible inflammation in the peribronchiolar region with few cells was observed in the normal lung section (Fig. 4A&B). Inflammatory cell influx in the peribronchiolar region was very prominent in acute asthma whereas modest inflammatory infiltrate was seen in the chronic case. The inflammation was enhanced fourfold (4 units) in acute asthma as compared to the normal which was higher than chronic asthma (3.19 units) as determined by the scoring system. Pre-treatment of curcumin (5mg/kg, i.n.) has effectively inhibited recruitment of inflammatory cells to the peribronchiolar region thereby reducing the inflammation up to 2.0 and 1.73 units in the acute and chronic models, respectively.
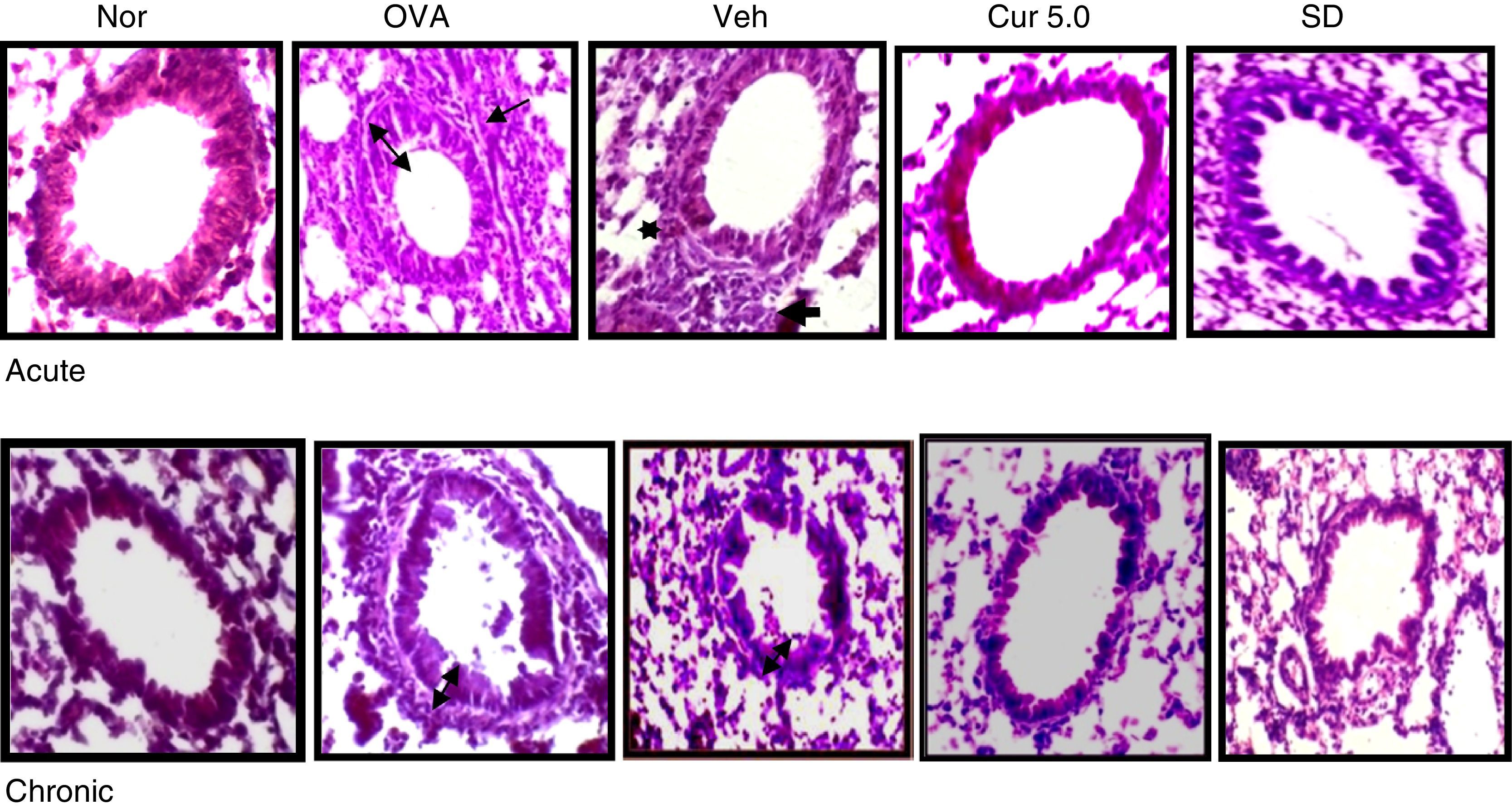
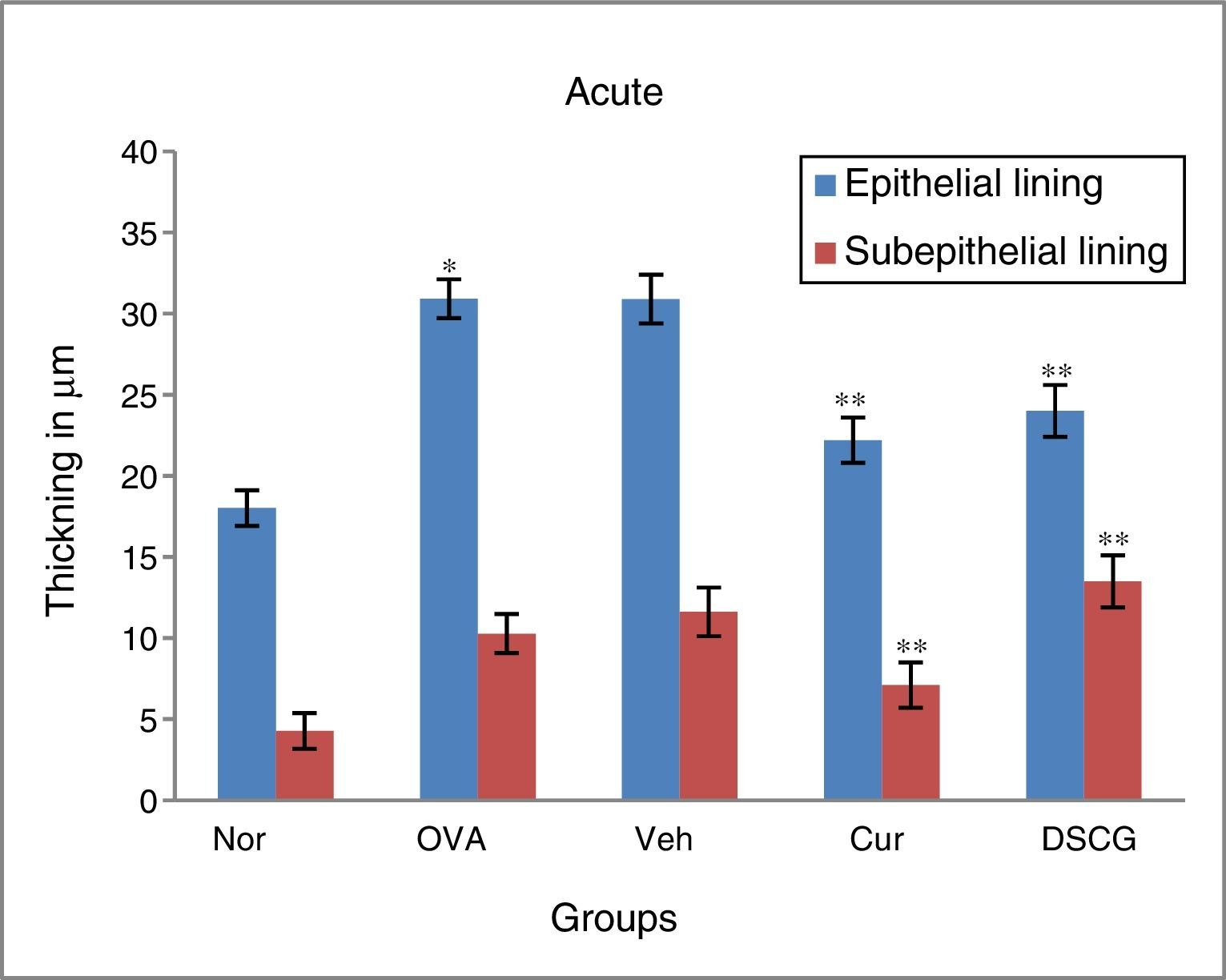
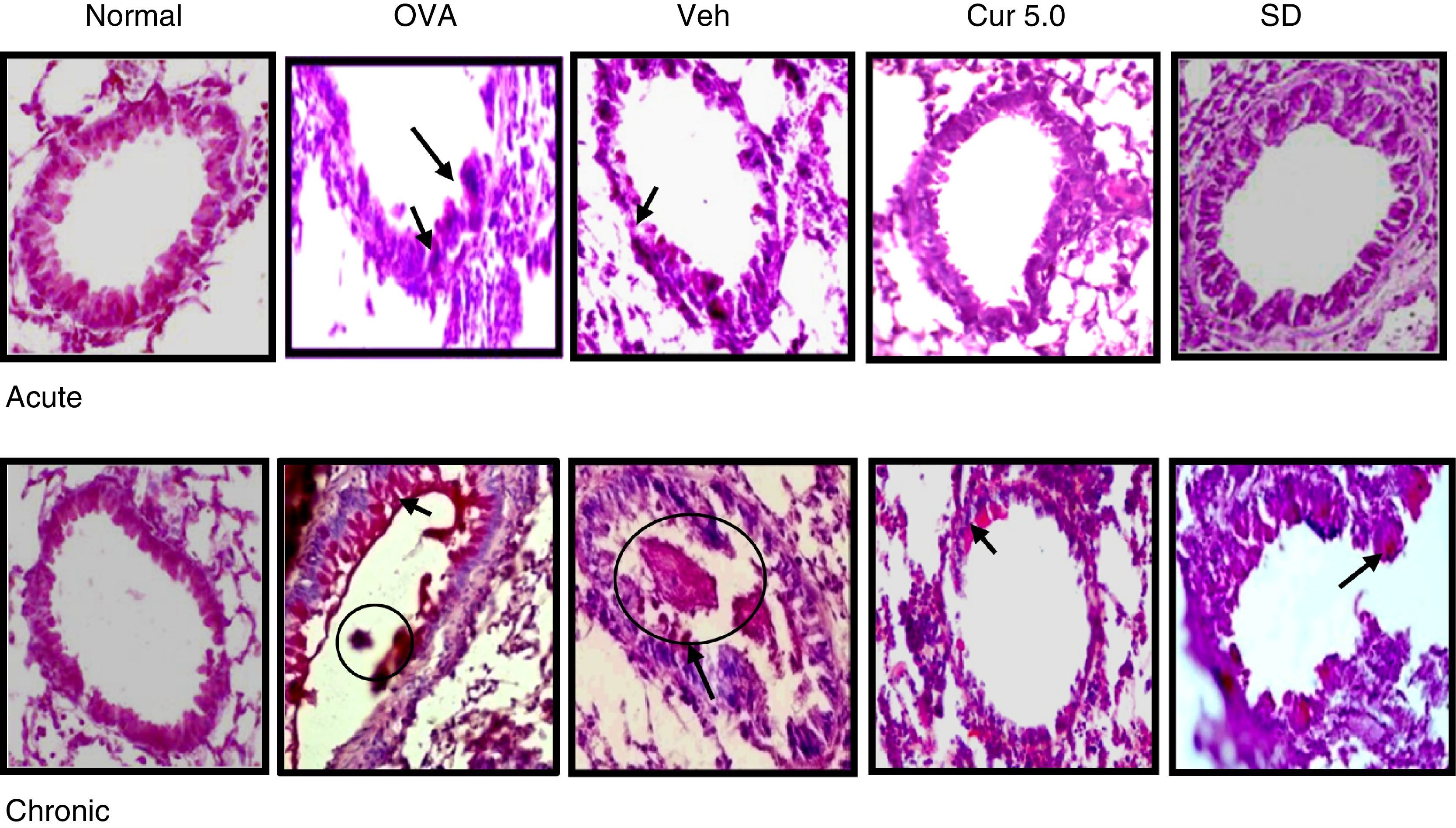
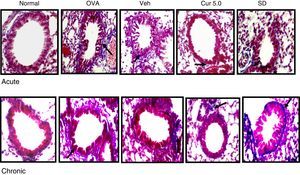
Effect of curcumin on airway inflammation and airway wall thickening 40×. Lung sections were stained with H&E and examined under light microscope. Epithelial and smooth muscle lining were measured at level of 12, 3, 6 and 9 o’clock position in 15–20 bronchioles. Epithelial and smooth muscle layers were thickened in OVA sensitised, challenged and vehicle-treated mice. The thickening was further reduced by curcumin administration in both the acute and chronic model. Single head arrows show inflammation and double head arrows show epithelial and subepithelial layer. Nor – normal; OVA – asthmatic; Veh – vehicle; SD – standard drug (DSCG in acute and dexamethasone in chronic).
Remodelling includes structural changes like epithelial and smooth muscle thickening, which are considered to be the main characteristic feature of chronic asthma. To measure the change in the epithelial and smooth muscle layers, H&E stained lung sections were used and measured at four point scale of each airway at levels of 3, 6, 9, and 12 o’ clock. It has been reported that structural changes occur in the chronic stage of asthma but sometimes repeated allergen challenge for few (3–4) consecutive days results in such changes in the acute phase itself (Fig. 5).
Effect of curcumin on peribronchial inflammation. 0–4 grade score system as described in materials and methods has been used to determine the peribronchial inflammation of airways. Curcumin significantly inhibited the peribronchial inflammation when compared with the asthmatic group both in acute and chronic16 cases. The results are shown in means±SEMs. *p<0.05 nor Vs OVA group and **p<0.05 OVA Vs cur group. Nor – normal; OVA – asthmatic; Veh – vehicle; SD – standard drug (DSCG in acute and dexamethasone in chronic).
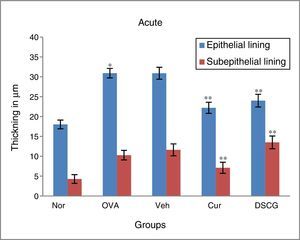
The epithelial and smooth muscle thickening in the OVA-exposed mice were increased both in the acute and chronic asthma as compared to the normal mice where no thickening was noticed. The epithelial and smooth muscle thickening was 30.93μm and 10.28μm respectively in acute mice, whereas it was enhanced up to 35.38μm and 12.39μm respectively in chronic condition.
Curcumin (5mg/kg) through nasal route was significantly effective in controlling the epithelial and smooth muscle thickening in both acute and chronic model of asthma. The epithelial thickening was reduced from 22.2μm to 18.02μm in acute condition which was near normal thickening and 25.71μm in chronic condition where normal thickening was 21.39μm (Fig. 6).
Effect of curcumin on epithelial and smooth muscle lining. In OVA-challenged group inflammation and thickening was found higher as compared to the control group in both acute and chronic16 models while in curcumin-treated mice both were reduced significantly as compared to the OVA group. Vehicle-treated group showed similar results like asthmatic mice. The results are shown in means±SEMs. *p<0.05 nor Vs OVA group and **p<OVA Vs Cur group. Nor – normal; OVA – asthmatic; Veh – vehicle; SD – standard drug (DSCG in acute and dexamethasone in chronic).
Mucus hyper secretion is an important symptomatic and pathological feature of a heterogeneous group of chronic respiratory diseases including chronic bronchitis, chronic obstructive pulmonary disease and asthma. For morphometric identification and measurement of goblet cells hyperplasia, PAS (Periodic acid) staining was performed and the magenta-coloured epithelial cells represent presence of goblet cells. Apart from the presence of goblet cells, magenta-coloured (mucin) mucus plug in bronchial lumen was also noticed after PAS staining.
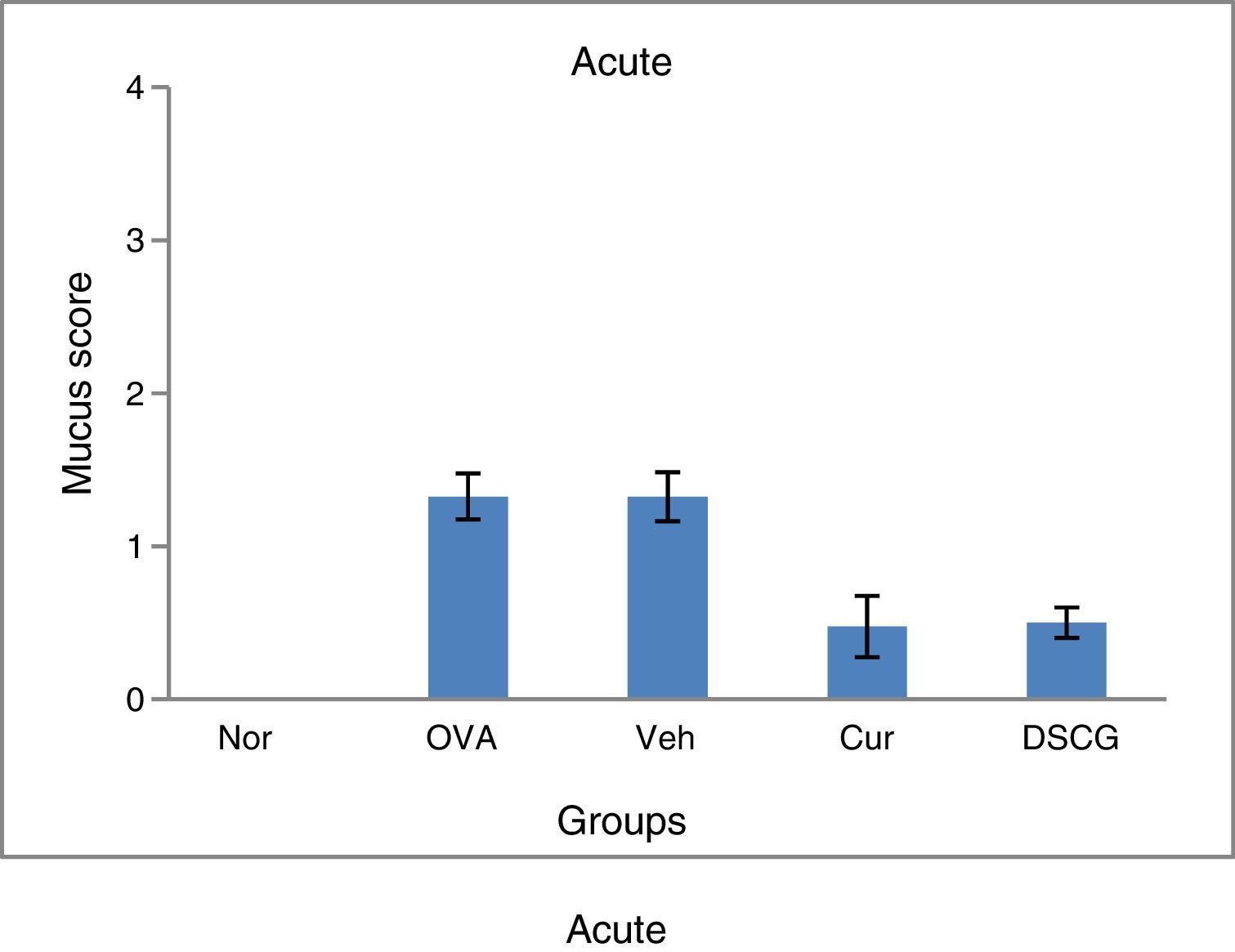
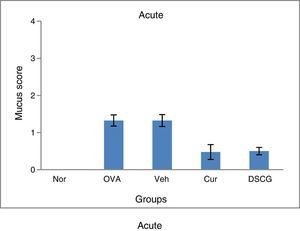
Curcumin treatment inhibited the goblet cell metaplasia thereby reducing goblet cell hyperplasia and mucin hyper secretion in airway lumen of bronchioles both in acute and chronic condition (Fig. 7). A consistent reduction in the mucus plug formation was also observed after curcumin treatment (5mg/kg) in chronic mice. In normal lungs 2–5% goblet cells were observed while OVA challenge resulted in 25% increase in acute asthma while maximum number of goblet cells and few epithelial cells could be seen in lung sections of chronic asthma (Fig. 8). Mucus plug formation was also noted in larger portion of the airway lumen in chronic model (Fig. 7). Goblet cell hyperplasia and mucus production was prominent mainly in larger and medium sized bronchioles as compared to small bronchioles.
Effect of curcumin on the goblet cell and mucus production 40×. For goblet cells and mucus production lung sections were stained with PAS and magenta coloured cells were identified and counted as goblet cells. Goblet cells were less in number with no mucus plug in acute asthma whereas large number of goblet cells with mucus plug in the lumen of airways was observed in the chronic model. Curcumin pre-treatment reduced the goblet cells in acute and chronic model along with the inhibition in the mucus plug formation in the chronic model. Arrows showing the goblet cells and circle marks the mucus plug. Nor – normal; OVA – asthmatic; Veh – vehicle; SD – standard drug (DSCG in acute and dexamethasone in chronic).
Effect of curcumin on the mucus production scored against PAS staining. Lung sections were stained with PAS and counterstained with Haematoxylin to detect mucus containing goblet cells. The mucus content in the cells was scored as 0–4 where 0 – no mucus-containing; 1 – few positive cells; 2 – few positive cells; 3 – numerous positive cells and 4 – numerous positive cells along the basement membrane. All scoring system was performed in at least 10–15 different fields. Chronic model has shown higher number of goblet cells as compared to the acute and intranasal curcumin effectively suppressed the goblet cells production in both the models.16 Results are shown in means±SEMs. *p<0.05 nor Vs OVA group and **p<0.05 OVA Vs cur group. Nor – normal; OVA – asthmatic; Veh – vehicle; SD – standard drug (DSCG in acute and dexamethasone in chronic).
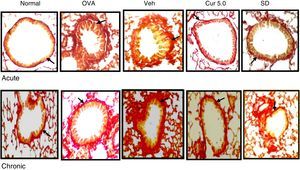
Extracellular matrix (collagen) deposition in the sub epithelial region is also a characteristic feature of repeated allergen challenge. Lung sections were stained with Masson's trichome and blue bands around peribronchiolar region of the airways were identified. Further, the deposition of collagen was also confirmed by Sirius red staining where the deep-red coloured band indicates the collagen deposition and was assayed by 0–3 scoring system in bronchi and 10–15 bronchioles were analysed for the study.
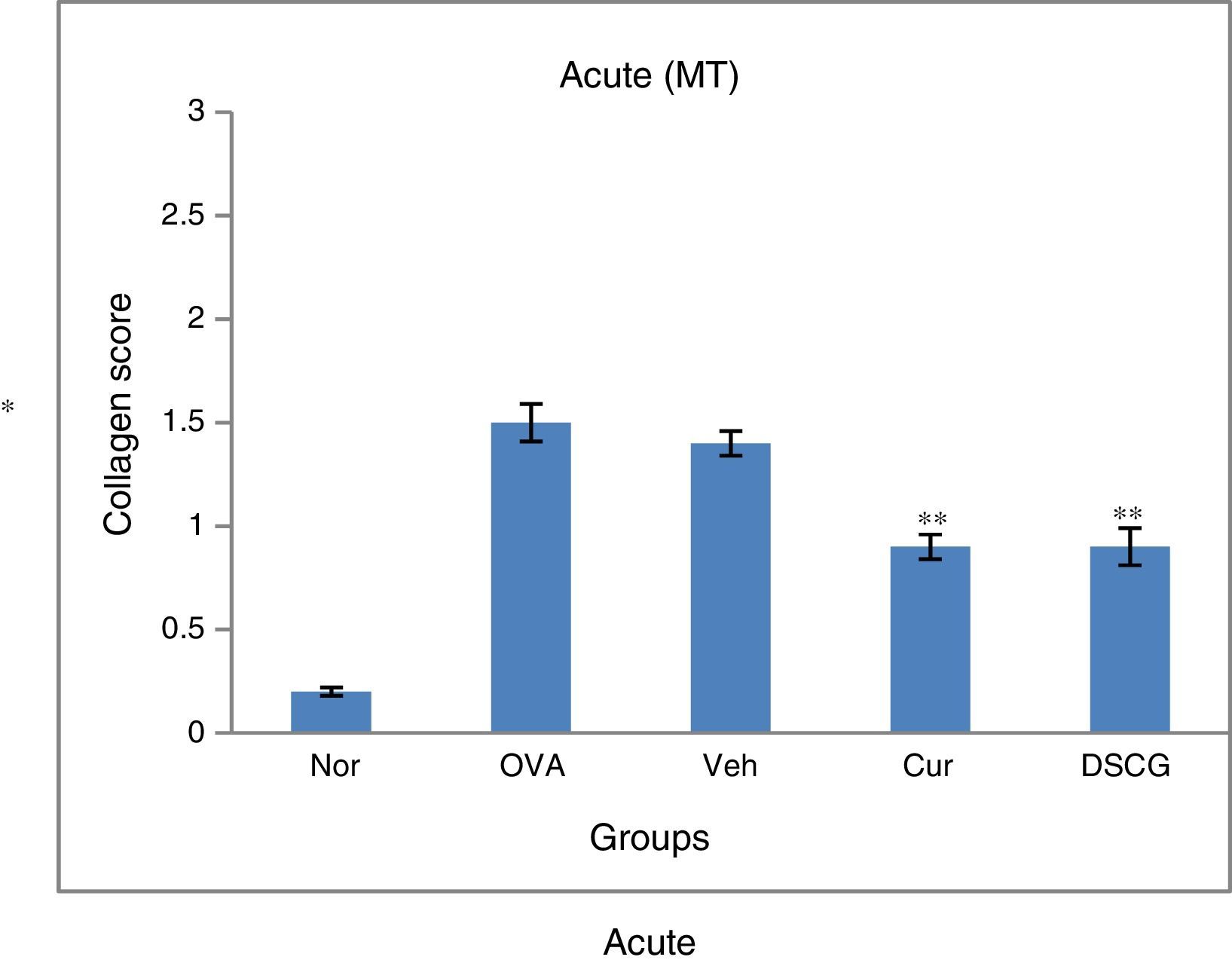
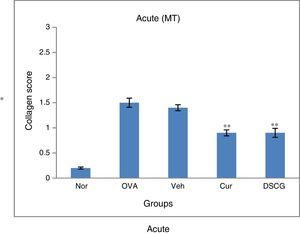
Both the asthmatic models, acute and chronic showed enhanced deposition of collagen in the sub-epithelial region around the peribronchiolar region as compared to the normal in Masson's trichrome stained slides (Fig. 9). In contrast, a significant increase in sub epithelial collagen deposition was evident in the chronic model as compared to the acute as per scoring system (Fig. 10). Curcumin (5mg/kg) treatment reduced accumulation of collagen around the peribronchiolar region in both the asthmatic models (Fig. 9). Further, collagen deposition was confirmed by Sirius red staining, where a consistent result was observed in the Masson's trichrome staining (Fig. 11A&B).
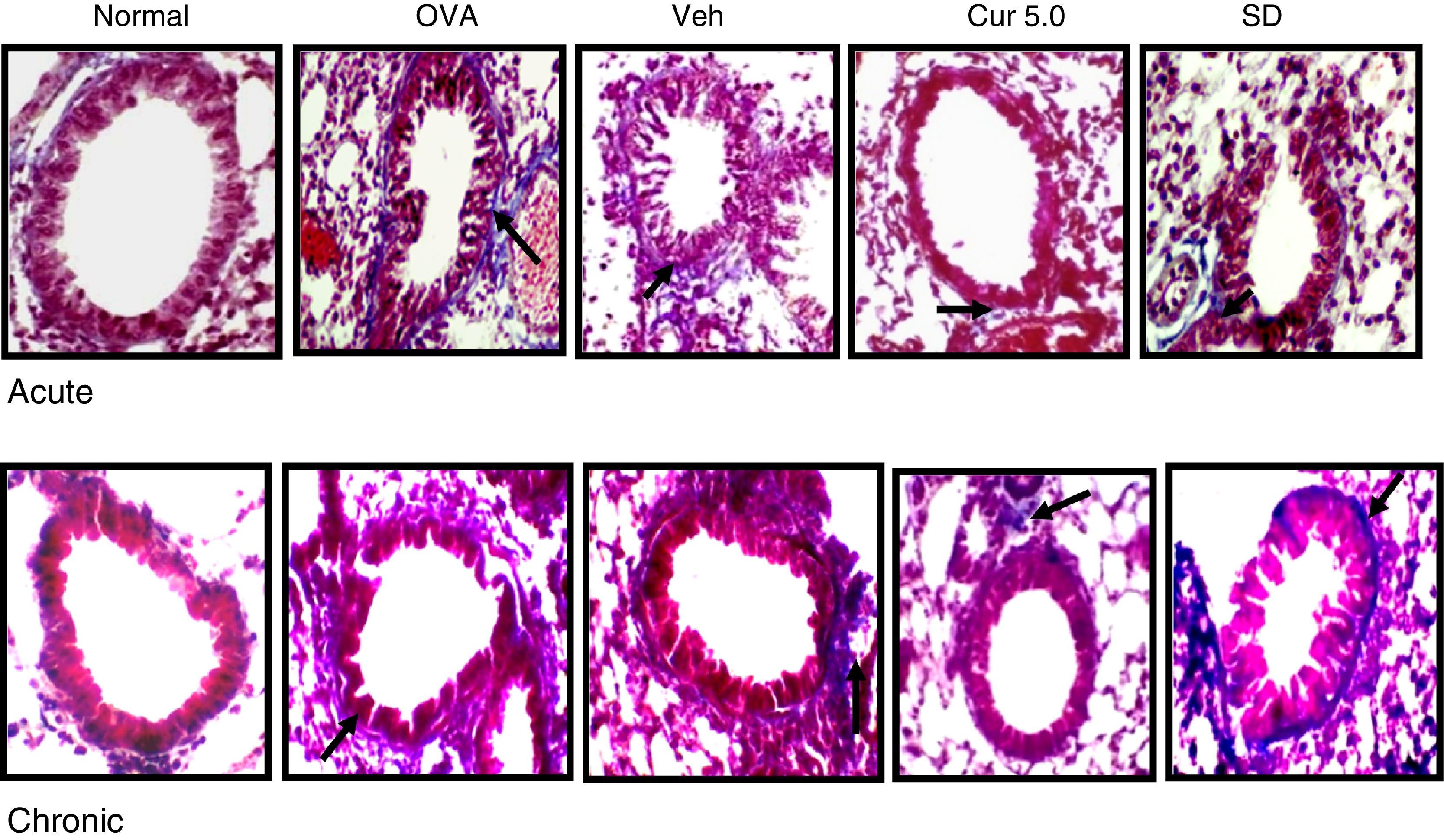
Effect of curcumin on collagen deposition in peribronchiolar region of lungs (40×). Collagen deposition was observed in lung sections by Masson's trichrome staining (10–15 peribronchiolar regions of airways has been viewed for evaluating blue band representing the collagen deposition). Collagen deposition was observed in the peribronchiolar region of both the acute and chronic models and curcumin intranasal administration inhibited the deposition in both. Chronic lung sections showed more deposition of collagen thereby destructing the peribronchiolar region. Arrows represent collagen accumulation around the peribronchiolar region. Nor – normal; OVA – asthmatic; Veh – vehicle; SD – standard drug (DSCG in acute and dexamethasone in chronic).
Effect of curcumin on the collagen deposition scored against Masson's trichrome staining. 0–3 scoring system has been performed to each bronchi where 0 – no collagen deposition; 1 – a thin layer of collagen; 2 – a cluster of collagen; and 3 – a thick layer of collagen. The scores obtained were graphed for acute and chronic16 model. Results are shown in means±SEMs. *p<0.05 nor Vs OVA group and **p<0.05 OVA Vs cur group. Nor – normal; OVA – asthmatic; Veh – vehicle; SD – standard drug (DSCG in acute and dexamethasone was used in chronic).
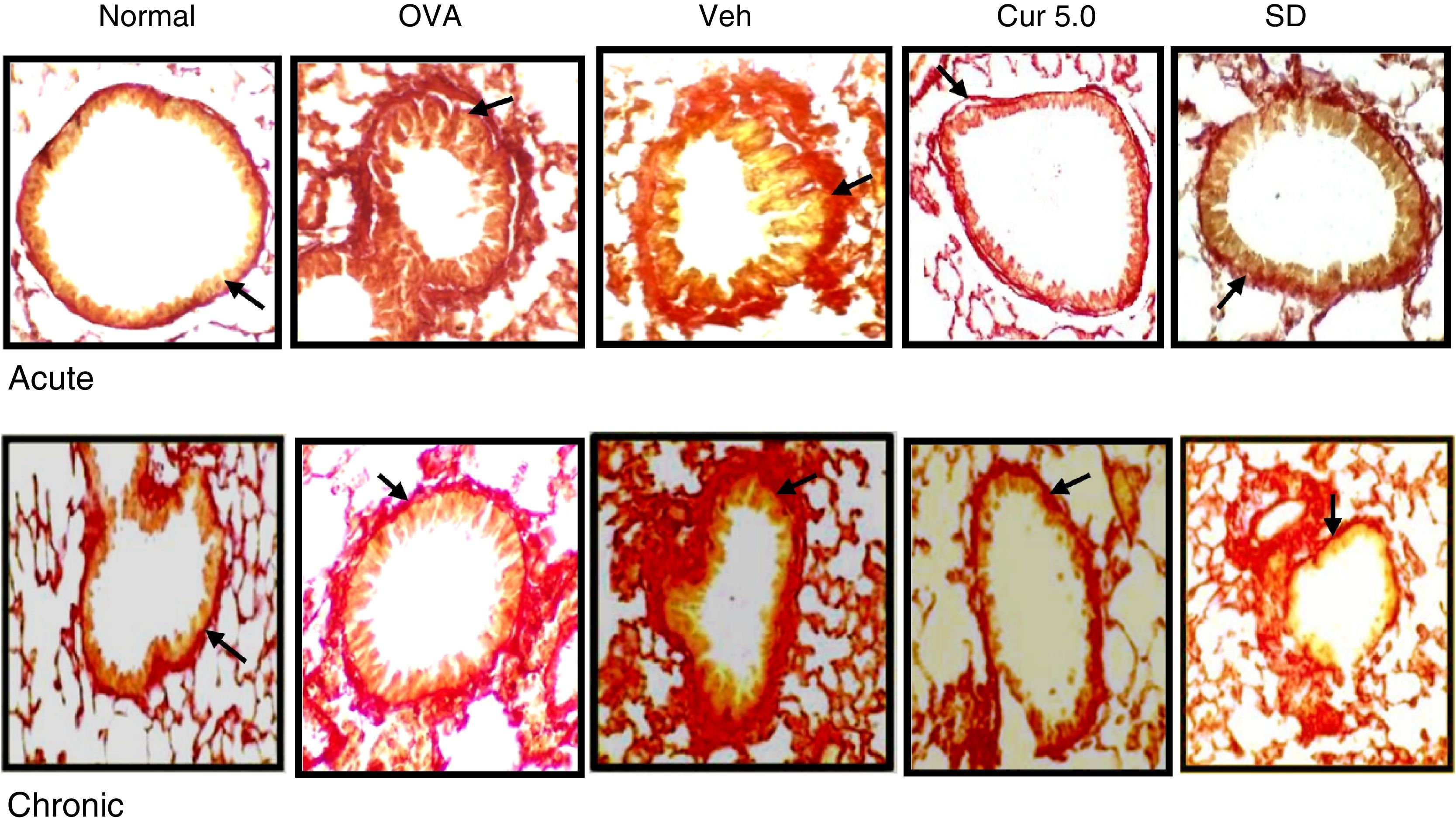
Effect of curcumin on collagen deposition confirmed by sirus red staining 40×: lung sections were stained by sirus red for collagen deposition. Presence of red band around smooth muscles indicates the collagen deposition. Acute model were observed with less collagen as compared to the chronic whereas curcumin-treated group showed thin lining of collagen in both acute and chronic. Arrows showing collagen deposition around bronchioles. Nor – normal; OVA – asthmatic; Veh – vehicle; SD – standard drug (DSCG in acute and dexamethasone in chronic).
The present study focuses on various structural changes leading to acute and chronic asthma in ovalbumin-induced mouse models and the effect of nasal curcumin (5mg/kg). Various aspects of sustained airway dysfunction, inflammation and remodelling, many of which were initiated in acute phase due to acute immune-mediated inflammatory events and persisted longer. Curcumin (5mg/kg, i.n.) has effectively inhibited the persistence of these structural changes in allergen (OVA)-induced mouse models of acute or chronic exposures.
Remodelling in asthma is recognised as a main pathological feature; however, the underlying physiological and immunological process and the mechanism regulating remodelling still need to be explored. Mouse models are being developed to reproduce asthma exacerbations and acute and chronic allergen challenge including ovalbumin are employed to study various characteristic features involved in its pathogenesis.22–26
Inflammation, as the main hallmark of asthma leads to remodelling by factors synthesised and secreted both by inflammatory cells and structural cells; the latter is frequently influenced by the former.27 Repeated exposure of OVA resulted in airway inflammation as a result of recruitment of cells to the lungs predominantly occupied by eosinophils along with lymphocytes, neutrophils and mast cells. As observed in the BALF and histological sections, the acute model showed severe inflammation as compared to the chronic model, suggesting that inflammation is the main characteristic feature of acute asthma. However, the enhanced total inflammatory cell count in BALF of acute mice but decreased to some extent in sections after prolonged exposure (chronic) but eosinophils were dominant over other inflammatory cells in both the models. Furthermore, the inflammation in peribronchiolar region of H&E lungs section was higher in the acute model as compared to the chronic, might be due to development of immune tolerance in chronic asthma with prolonged exposure. Hence, enhanced inflammation is not seen after prolonged exposure. Curcumin administered at 5mg/kg through nasal route was found effective in significantly inhibiting the recruitment of total cells along with eosinophils to the airways and also inhibited the peribronchiolar inflammation in both acute and chronic groups. The inhibitory effect of curcumin was comparable to the standard drug as DCSG (acute) and Dexa (chronic). No inhibitory effect of vehicle (di methyl sulphoxide) was observed in both the models.
Repeated OVA challenge (from 3 days to several weeks) results in several structural alterations in the airways termed as “airway remodelling” which occurs as a result of an injury/repair process. A number of studies confirm numerous structural changes as airway wall thickening, subepithelial fibrosis around bronchioles, goblet cell hyperplasia and hypertrophy, mucus gland hyperplasia/metaplasia with mucin production in airway lumen, hyperplasia and hypertrophy of myofibroblast and epithelial cells as part of airway remodelling.28,29 These are not only seen in severe asthma but also in mild and moderate asthma and hence are now being focused as a therapeutic target.30,31
Epithelial cells act as key structural tissue and are the first line of defence against exposure of the airway and lung to inflammatory stimuli/antigens via its activation. In the present study, structural changes such as epithelial and sub-epithelial thickening, collagen deposition along with goblet cells manifestation were observed as early structural changes in the course of airway remodelling in acute asthma. Epithelial and subepithelial thickening were evident after just 4 days of OVA inhalation and persisted till the chronic condition but this thickening seems to be more prominent in chronic asthma as compared to acute. Further, the chronic model illustrates some peculiar changes such as epithelial injury leading to damage and shedding off epithelial cells along with its thickening but no such injury or damage was observed in the acute model. Damage to the epithelial layer might represent epithelial cell apoptosis due to repeated injury for longer. Intranasal curcumin administration significantly inhibited epithelial and sub-epithelial layer thickening in both the models of asthma. It also has inhibited the epithelial cell injury, thereby reducing airway damage in the chronic asthma.
Matrix metalloproteinases (MMP-2 and MMP-9) degrade basement membrane components and, therefore, facilitate regress of inflammatory cells, and also counteract airway wall leading to fibrosis.32 Myofibroblasts, which have a mixed contractile and collagen synthesising phenotype, are likely to participate in subepithelial deposition of collagen and other matrix proteins that cause classical thickening of the lamina reticularis. Strikingly enhanced collagen deposition in the sub-epithelial thickening around the peribronchial layer was observed in both models as determined by morphometric analysis in Masson's trichrome and Sirius red stained lung sections which is consistent with previous reports.33 Intranasal curcumin administration inhibited the deposition of collagen in both the asthma models.
Phenotypic changes in the epithelium leads to goblet cell metaplasia/hyperplasia and a subsequent oversecretion of mucus are well known features of remodelling in asthma. Moreover, mucus plug generation in the airway lumen are major contributors to asthma fatalities which together with the bronchial gland enlargement, leads to mucus hyper secretion eventually causing airway obstruction.34 These features are reported to be prominent in some animals between 24 and 36h after a single exposure to antigen but information on the role of goblet cell hyperplasia is less conclusive in mild and moderate asthma.35,36 We found here, short exposures of OVA (4 days) were sufficient to elicit minor goblet cells with little mucus secretion in acute phase, whereas abrupt goblet cells were observed after long OVA exposures. These goblet cells hyperplasia are the main contributor of epithelial thickening and hence prominent epithelial thickening was observed in chronic as compared to acute model. Interestingly, goblet cell hyperplasia led to the formation of mucus plugs encompassing the whole airway lumen in chronic asthma which was not seen at all in the acute phase. Curcumin inhibited the goblet cell hyperplasia along with the reduction in the mucus secretion in the chronic model. Minor goblet cells seen in the acute phase were also reserved after curcumin treatment. Reduction in the goblet cell hyperplasia resulted in the reduction of mucus plug in the lumen of airway eliciting the capability of curcumin where less than 5% epithelial cells have transformed to goblet cells.
The present study implies that acute-onset of allergic asthma is distinct from chronic somewhere in terms of structural alteration (mainly goblets cells hyperplasia, mucus plug, epithelial shedding). Administration of intranasal curcumin (5mg/kg) ameliorates structural changes in acute as well as chronic asthma. We have reported for the first time that nasal route revealing the improved absorption of curcumin in the lungs which are directly targeted without being degraded and this could be a strong reason for elucidating the maintenance of structural changes.16
Besides having anti-inflammatory and anti-asthmatic potential, intranasal curcumin is effective in maintaining the structural integrity of the lungs in chronic asthma as well. Molecular mechanism of curcumin action in maintaining the structural integrity is in progress that can prove it as better drug of choice to treat asthma without any side effect even if taken for longer duration.
Ethical disclosuresPatients’ data protectionThe authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
Protection of human subjects and animals in researchThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Conflict of interestAll the authors declare no conflict of interest.
The authors are thankful to University Grants Commission and Science and Engineering Research Board, Department of Science and Technology (SERB-DST), New Delhi, India in part for financial assistance.