Asthma is an inflammatory disorder of the airways associated with bronchial hyperresponsiveness, airway obstruction, and increased mucus production, with a predominance of type 2 immune response (Th2). According to the hygiene hypothesis, exposure to environmental bacterial lipopolysaccharide (LPS) may induce a type 1 immune response (Th1), modulating the development of asthma.
ObjectiveIn this study we investigated cytokine production by peripheral blood mononuclear cells (PBMC) from children and adolescents with severe asthma, in response to LPS stimulation in vitro.
Materials and methods26 children were selected: 13 severe asthmatics and 13 healthy controls, aged between 5 and 18 years. They were evaluated through routine medical history, physical examination and lung function test to diagnose severe asthma. Allergy status was confirmed by skin prick test and specific IgE assay. We collected blood samples to analyse in vitro LPS-induced cytokines release by PBMC.
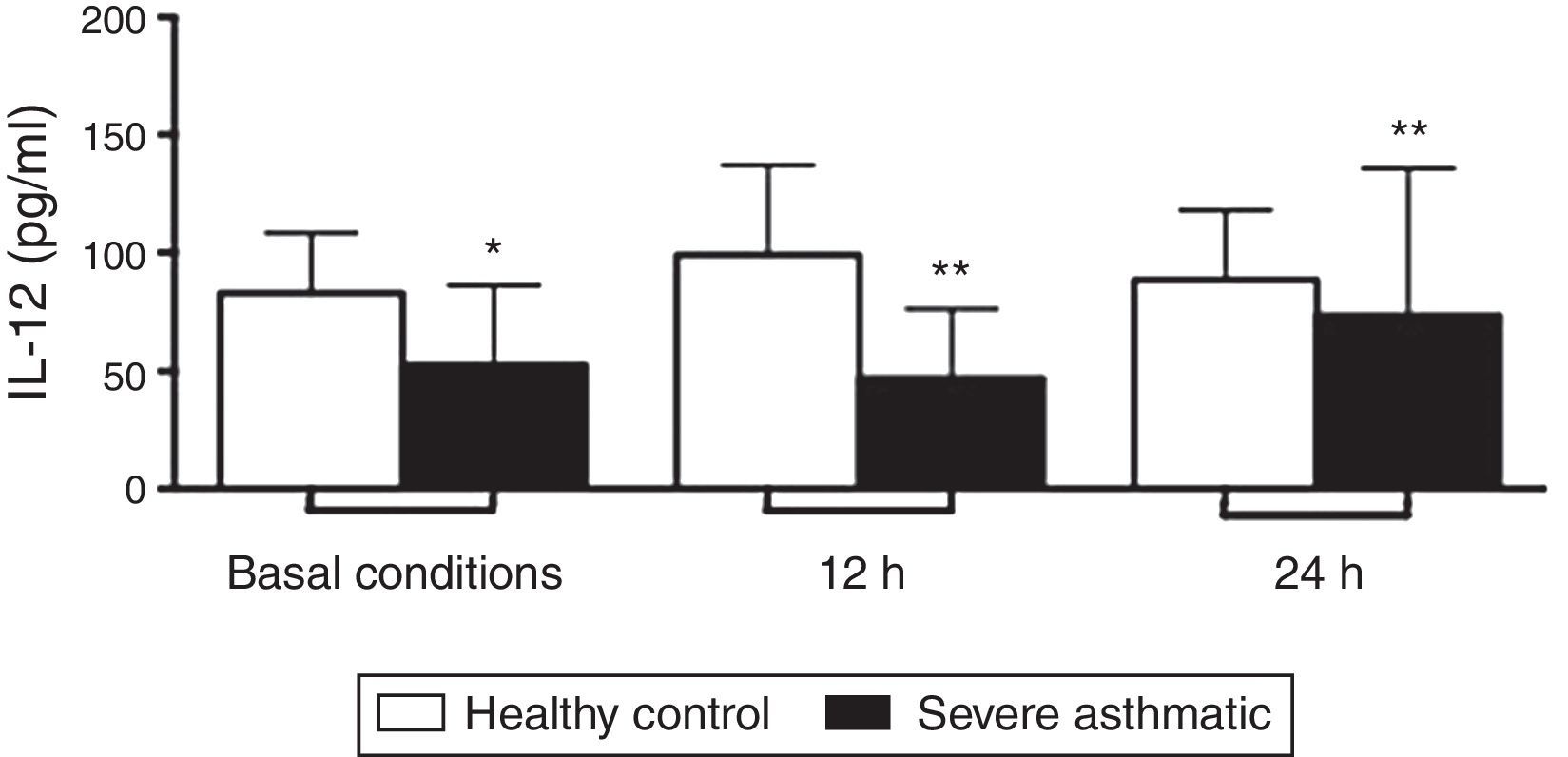
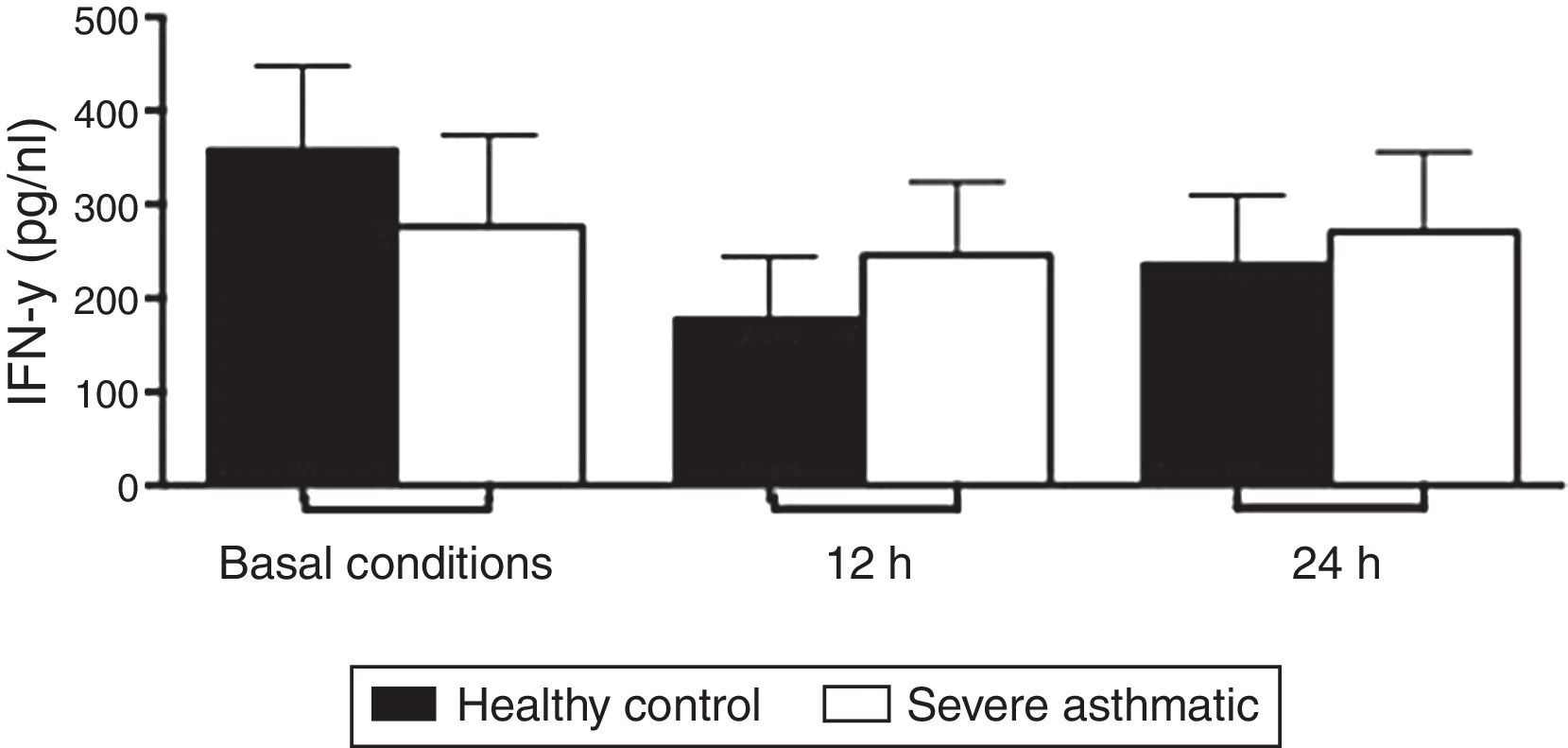
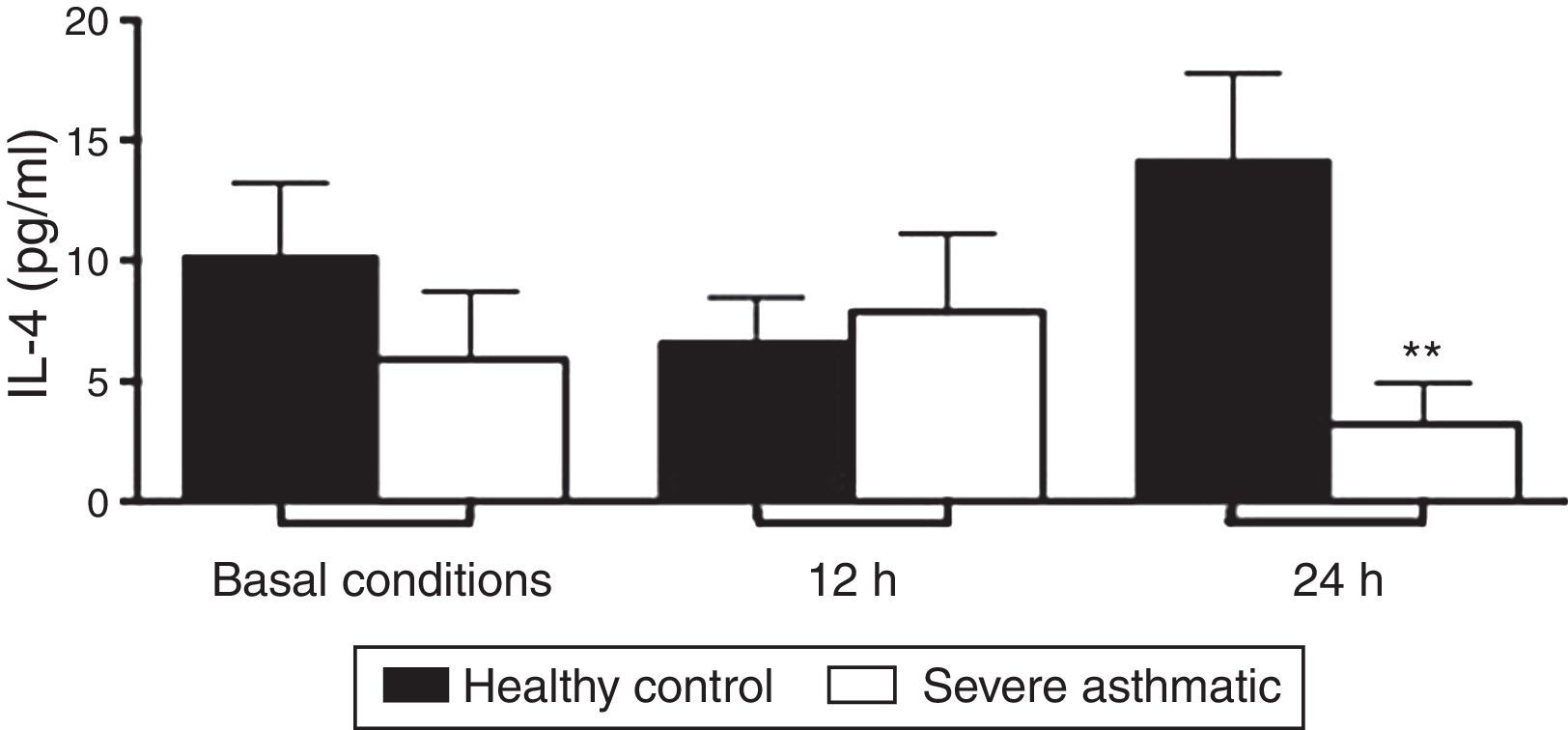
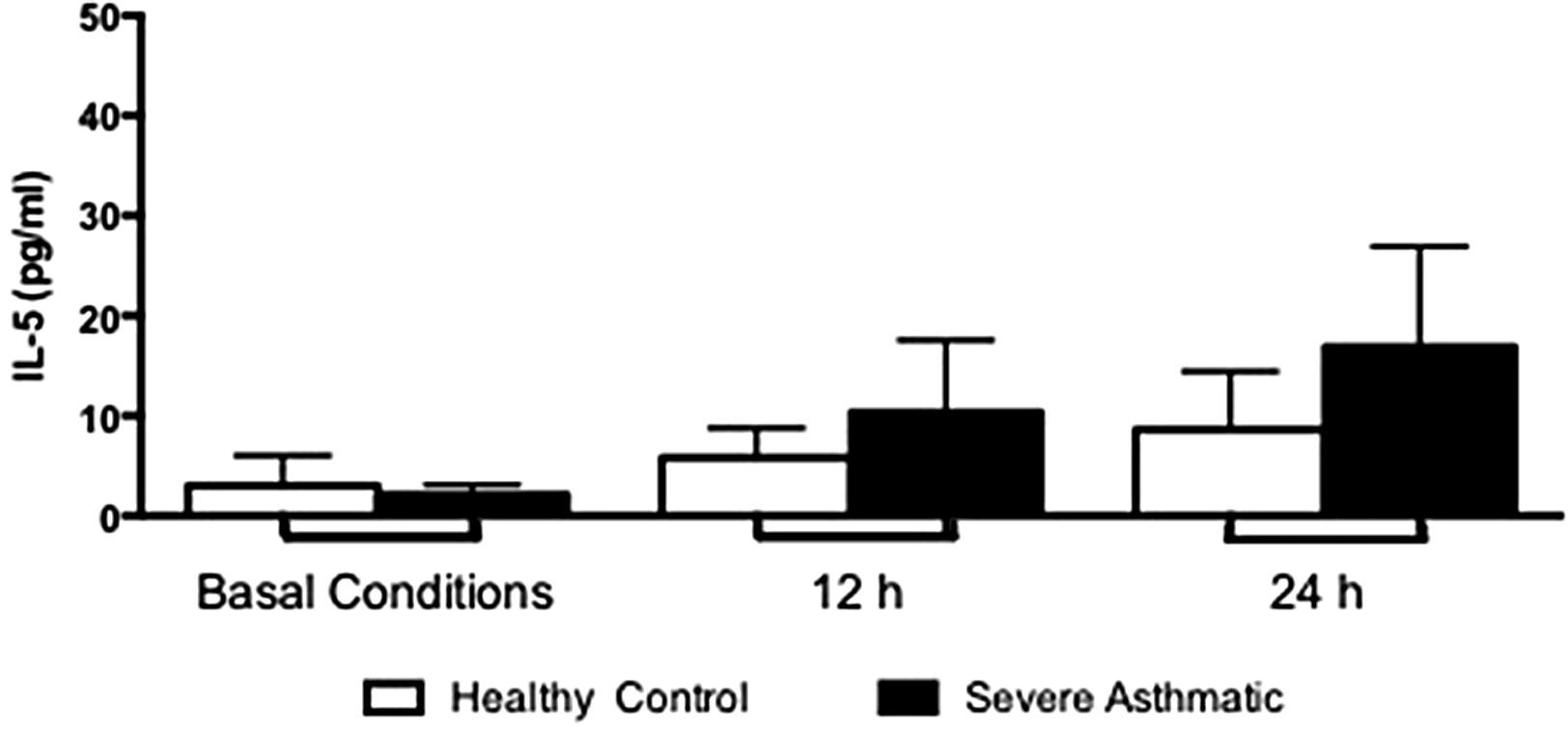
ResultsPBMC from severe asthmatic children produced lower levels of IL-12p70 in basal conditions and after 12 and 24h stimulation with LPS compared to healthy controls. PBMC from severe asthmatic children produced lower levels of IL-4 after 24h LPS stimulation compared to healthy controls. PBMC from severe asthmatic children produced more levels IL-17 and IL-10 after stimulus with LPS compared to healthy controls. The release of IFN-γ, IL-5 and TNF-α by PBMC from severe asthmatic children was similar to healthy controls.
ConclusionOur results demonstrate that LPS directly influence the cytokine profile of PBMC in children with severe asthma. These observations may be potentially helpful in developing new treatment strategies.
According to the World Health Organization over 300 million people are affected by asthma worldwide, 60% are children, and Brazil is the eighth country in the prevalence of this pathology.1 These epidemiological studies suggest an increased prevalence of asthma in the developed world in recent decades, especially in children and teenagers.2,3
The allergic predisposition can be attributed to alterations in the fine balance between type 1 helper T lymphocytes (Th1) and type 2 helper T lymphocytes (Th2) responses towards a stronger Th2 pattern.4 Evidence has extensively shown that activated Th2 lymphocytes and cytokines such as interleukin (IL)-4, IL-13, and IL-5 are responsible for the cascade of eosinophil activation and IgE production necessary for allergic inflammation.5 However, recent studies demonstrated that other immune responses, such as Th1 (IL-12 and IFN-γ), regulatory (IL-10), and inflammatory (IL-17 and TNF-α), might be involved in the development of asthma.6,7
Kondo et al.8 showed the reduced IFN-γ production by antigen-stimulated cord blood mononuclear cells as a risk factor to develop allergic disorders, as dermatitis and asthma. In 2003, Guerra et al.9 in a longitudinal study showed that impaired IFN-γ production by peripheral blood mononuclear cells (PBMC) at three months of age significantly increases the risk of developing recurrent wheezing in the first year of life. More recently, our group demonstrated that LPS-stimulated PBMC from recurrent wheezing (RW)-children exposed to environmental endotoxin above 50EU/mg release lower levels of IL-12 and IFN-γ.10 The study of those different immune patterns can characterise novel wheezing phenotypes not yet observed. In this work we aimed to evaluate the LPS-induced cytokines production profile by peripheral blood mononuclear cells (PBMCs) from severe asthmatic children at 5–18 years of age compared to healthy controls.
Materials and methodsSubjects and study designThirteen healthy controls and thirteen severe asthmatic children aged 10–18 years were recruited from the Division of Pediatric Allergy-Immunology and Rheumatology at Federal University of São Paulo Medical School. Clinical asthma was diagnosed according to GINA (Global Initiative for Asthma) criteria, through routine physical examination and applying a questionnaire to the parents or legal guardians. Spirometric measurements were performed as described,11,12 and severe asthma was confirmed.
We also performed skin prick tests and collected peripheral blood for in vitro assays at the Center of Human Immunology, University of São Paulo, Brazil. All study procedures were carried out in accordance with a previously approved protocol by the Ethics Committee of the Institute of Biomedical Sciences, University of São Paulo, according to Brazilian Ministry of Health (resolution 196/96). All parents provided written informed consent for the study procedures.
Skin prick test (SPT)All individuals underwent SPT with the following allergens extracts: Dermatophagoides pteronyssinus, Dermatophagoides farinae, Blomia tropicalis, Blatella germanica, Periplaneta americana, Felix domesticus, Canis familiaris, rat, mouse, fungi, pollen, cow milk, egg, and soybean (IPI-ASAC, São Paulo, Brazil). Histamine (1mg/mL) and saline were positive and negative controls, respectively. A mean wheal 3mm in diameter larger than the negative control was considered to be positive.
Cell isolation and culture conditionsPeripheral blood mononuclear cells (PBMC) were isolated from heparinised venous blood by density-gradient centrifugation on Ficoll-Hypaque 1077, as described previously.13 Cell viability was over 90% as assessed by Trypan blue staining (Sigma Chemical Co.).
1×106PBMC/well were cultured in 1mL of DMEM containing 5% AB+ human serum, 2mM l-glutamine, 100U/mL penicillin, and 10mg/mL streptomycin, In 24-well plates, in the absence or presence of lipopolysaccharide (LPS; 10μg/mL) (Escherichia coli 026:B6; SIGMA BioXtra, St. Louis, MO, USA), at 37°C in humid atmosphere saturated with 5% CO2, during 12 and 24h. Supernatants were collected at each time for cytokine determination.
Cytokine production assay – ELISAConcentrations of interleukin 12 (IL-12p70), interferon-gamma (IFN-γ), and interleukin 4 (IL-4), were assessed using commercially available Pelikine Compact ELISA Kits (CLB, Amsterdam, Netherlands). Detection ranges were: IL-12p70 (4±8pg/mL); IFN-γ (4±8pg/mL); IL-4 (0.5±0.6pg/mL). Assays were performed according to the manufacturer's instructions. All measurements were carried out in duplicate with a <10% variation between the two measurements.
Statistical analysisStatistical analysis was performed using GraphPadPrism 5.0 (GraphPad Software, CA, USA). We have performed 2×2 contingency tables, chi-square, and Fisher's test with Yates correction, with a 95% interval. Non-parametric Mann–Whitney test was used to compare data without Gaussian distribution. Correlation analysis was determined using the non-parametric Spearman's test. Differences were considered to be statistically significant when p<0.05.
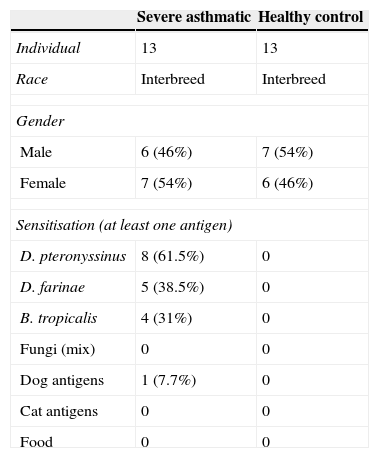
ResultsClinical outcomeThe demographic and clinical characteristics of the study subjects are reported in Table 1. The study included 26 children aged 10–18 years; 13 with severe asthma, and 13 healthy controls. All the children were tested for SPT and all healthy controls were non-allergic. SPT results of children with severe asthma showed that 61.5% patients had positive SPT to D. pteronyssinus, 38.5% to D. Farinae, and 31% to B. tropicalis. All severe children performed spirometric measurements and treatment with β2-agonists.
Clinical profile of severe asthmatic children and healthy controls.
| Severe asthmatic | Healthy control | |
|---|---|---|
| Individual | 13 | 13 |
| Race | Interbreed | Interbreed |
| Gender | ||
| Male | 6 (46%) | 7 (54%) |
| Female | 7 (54%) | 6 (46%) |
| Sensitisation (at least one antigen) | ||
| D. pteronyssinus | 8 (61.5%) | 0 |
| D. farinae | 5 (38.5%) | 0 |
| B. tropicalis | 4 (31%) | 0 |
| Fungi (mix) | 0 | 0 |
| Dog antigens | 1 (7.7%) | 0 |
| Cat antigens | 0 | 0 |
| Food | 0 | 0 |
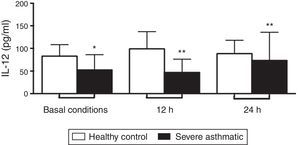
PBMC from severe asthmatic children produced lower levels of IL-12p70 in basal conditions, and after 12 and 24h stimulation with LPS compared to healthy controls (Fig. 1, Mann–Whitney test, p<0.05).
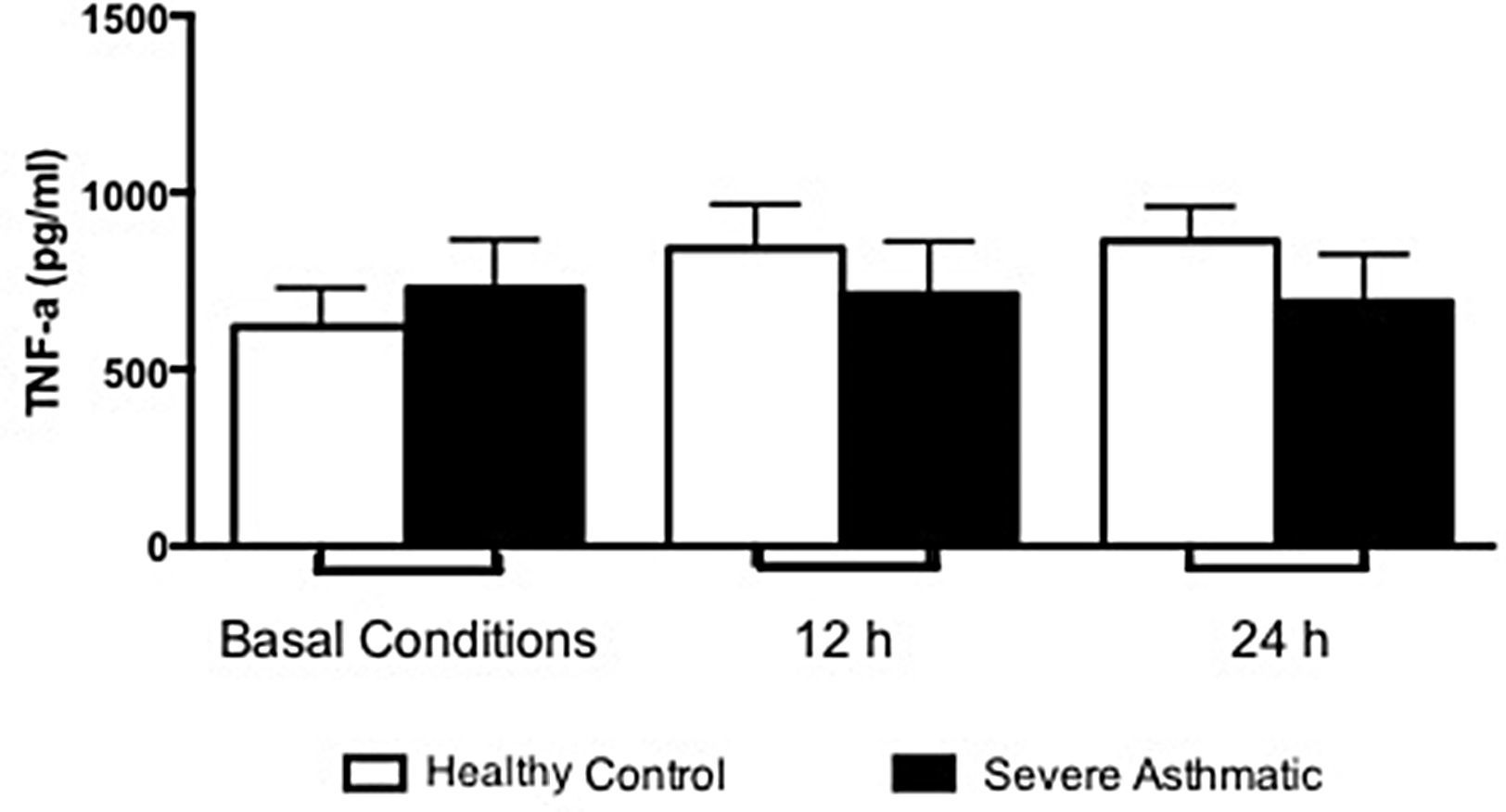
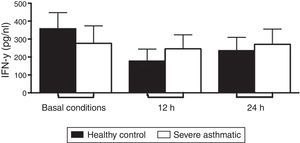
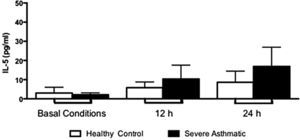
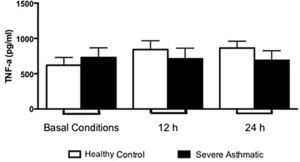
The IFN-γ, IL-5 and TNF-α production by PBMC from severe asthmatic children and healthy controls was similar at both basal conditions or after 12 and 24h stimulation with LPS (Fig. 2, Mann–Whitney test, p>0.05; Supplementary Figs. 1 and 2, Mann–Whitney test, p>0.05).
Supplementary Figures 1 and 2 related to this article can be found, in the online version, at http://dx.doi.org/10.1016/j.aller.2014.10.005.
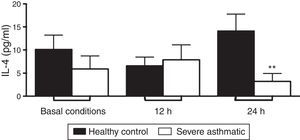
PBMC from severe asthmatic children produced lower levels of IL-4 after 24h stimulation with LPS compared to healthy controls (Fig. 3C, Mann–Whitney test, p<0.05). At basal conditions and after 12h stimulation with LPS, PBMC from severe asthmatic children produced similar amounts of IL-4 compared to healthy controls (Fig. 3A and B; Mann–Whitney test, p<0.05).
IL-4 production by PBMC of severe asthmatic children. PBMC from severe asthmatic children produce similar levels of IL-4 at basal conditions, after 12h stimulation with LPS (p>0.05 in all situations, n=13, Mann–Whitney test). After 24h stimulation with LPS, PBMC from severe asthmatic children produce lower levels of IL-4 compared to healthy controls (*p<0.05, n=13, Mann–Whitney test).
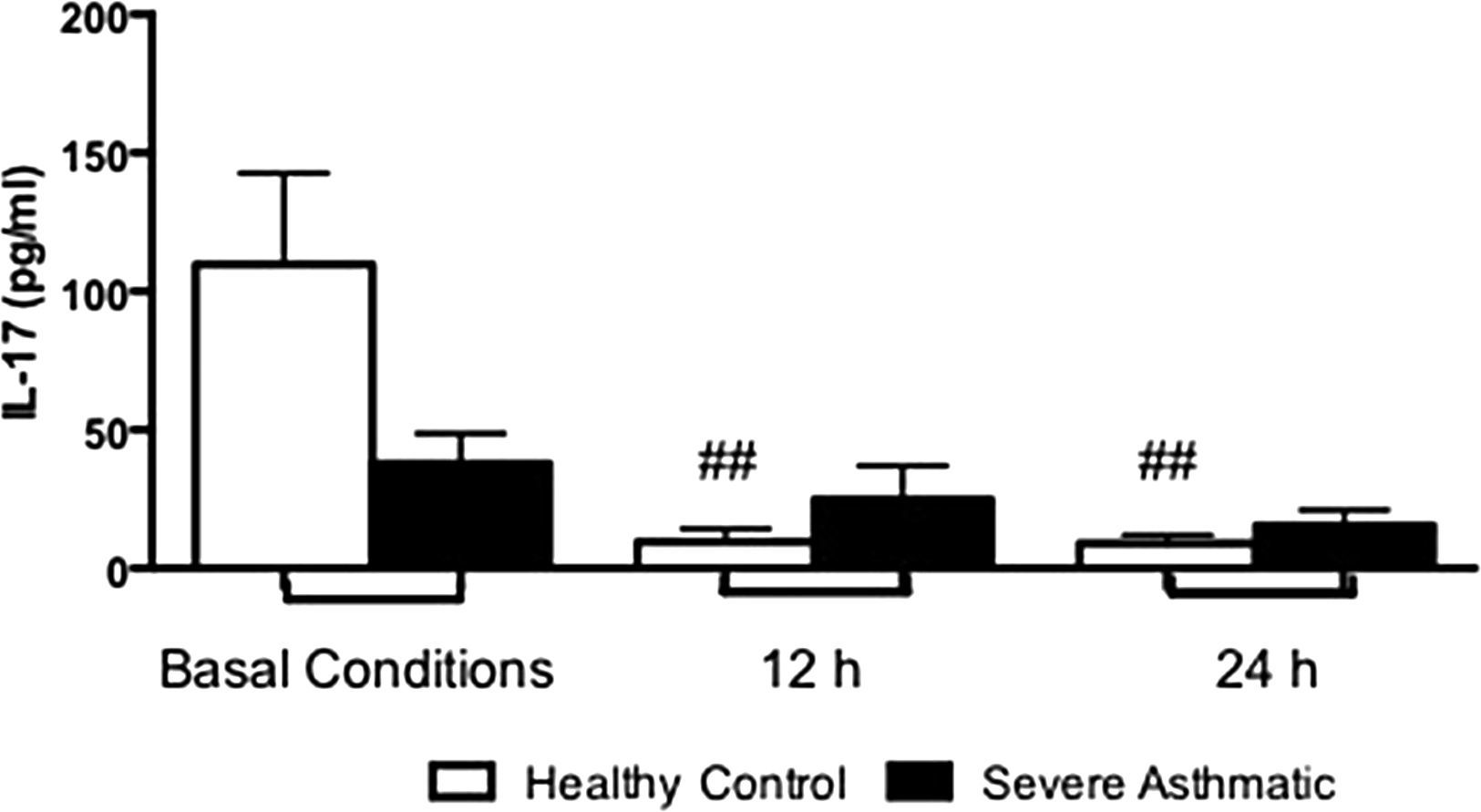
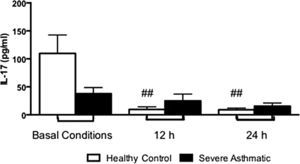
PBMC from severe asthmatic children produce more levels of IL-17 after 12 and 24h stimulation with LPS compared to healthy controls (Supplementary Fig. 3, Mann–Whitney test, p<0.05).
Supplementary Figure 3 related to this article can be found, in the online version, at http://dx.doi.org/10.1016/j.aller.2014.10.005.
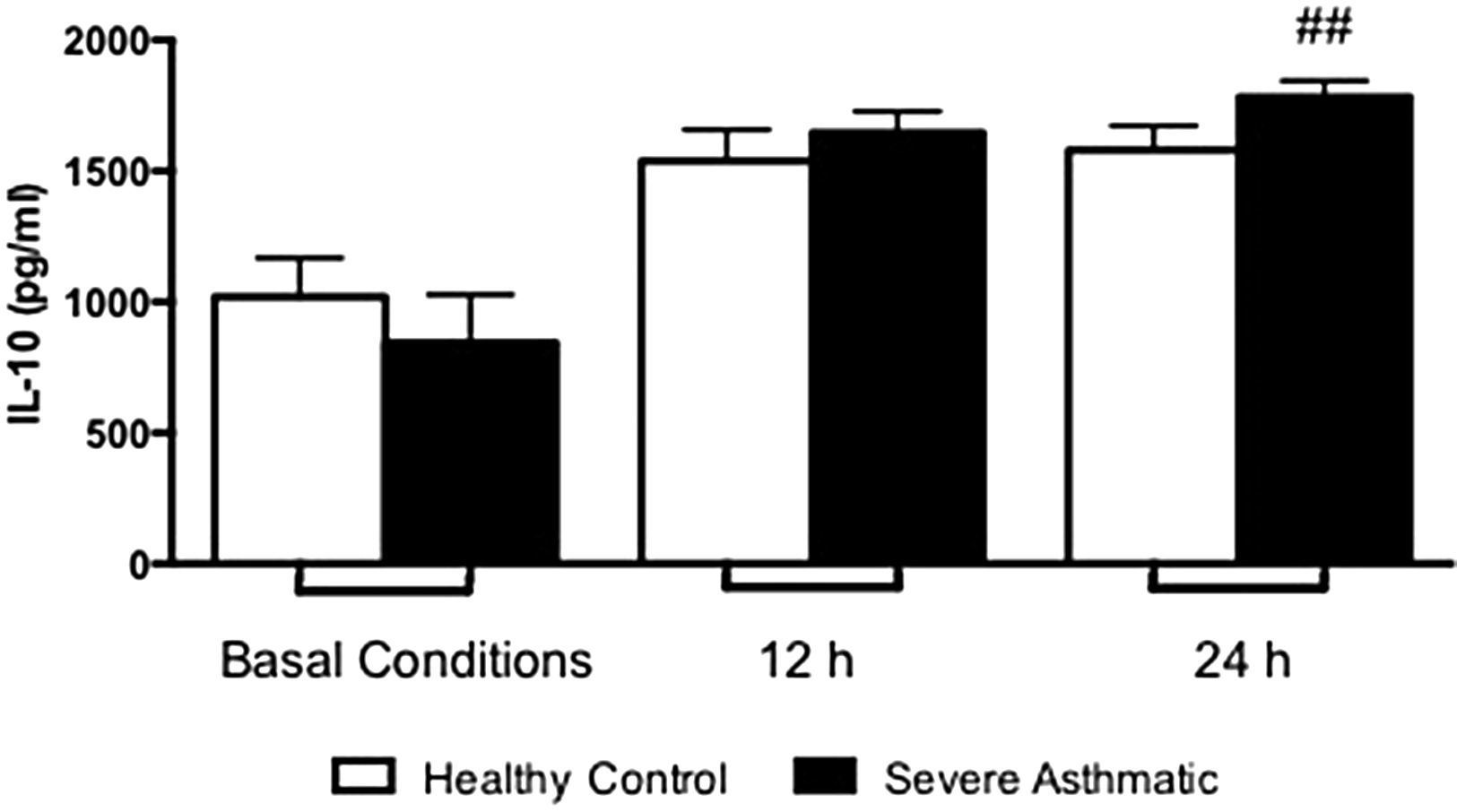
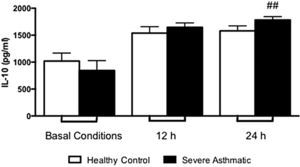
The IL-10 production by PBMC from severe asthmatic children produce higher levels of IL-10 after 24h stimulation with LPS compared to healthy controls (Supplementary Fig. 4, Mann–Whitney test, p>0.05). At basal conditions they produced similar amounts of IL-10 between PBMC from severe asthmatic children and healthy controls.
Supplementary Figure 4 related to this article can be found, in the online version, at http://dx.doi.org/10.1016/j.aller.2014.10.005.
DiscussionThe immune responses on asthma involve T lymphocytes activation – after antigen presentation, but differentiation between Th1, Th2, Th17 or Treg pattern is influenced by several factors, such as the nature of the antigen, costimulatory signals and cytokine profile of the environment.14–16 Thus, while IL-4 induces differentiation to Th2 cells, IL-12 induces Th1 differentiation.17 In the immunopathogenesis of asthma the role of Th2 cells is well known. Asthmatic subjects have a greater number of allergen-specific T cells secreting IL-4 and IL-5.18 In this work, we show a lower production of IL-4 by LPS-stimulated PBMC from severe asthmatic children. These results provide new evidence corroborating with recently published research demonstrating that low proliferative response to LPS and IL-4 production may constitute a risk factor for the development of asthma and a well-defined feature in patients with established asthma.
We show that PBMC from severe asthmatic children produce lower amounts of IL-12p70 in basal conditions. This defect was also evident after 12 and 24h stimulation with LPS, when IL-12p70 presented lower amounts in cultures from asthmatic cells compared to healthy controls. However, IFN-γ release by PBMC from severe asthmatic children was similar to healthy controls levels. These observations corroborate with other studies showing that the Th1 response could be impaired in allergic children.19 Those results highlight the defects in Th1 response and bring novel perspectives regarding the Th1/Th2 paradigm in atopy and asthma.20
Beyond all those other group observations, the data from our study reinforce the suspicion that low spontaneous production of Th1 cytokines could be a permanent, rather than transient, defect of PBMC from patients with wheezing and asthma. Still, this “failure” is shown independent of early exposure to endotoxin, increasing the possibility of a genetic defect in molecules and pathways involved in the activation of the IL-12/IFN-γ axis.10 Following those results, we can suggest that the imbalance in IL-12/IFN-γ axis is an intrinsic defect related to IL-12 production, showing no direct relationship with IFN-γ release. Compensation mechanisms are probably involved in this process, promoting the maintenance of IFN-γ levels, as IL-18 production that contributes to inducing IFN-γ in an immunosuppressive environment.21
Other cytokines were analysed in order to understand the mechanisms that lead to asthma development. In the context of our treatment protocols, we evaluated peripheral blood monocytes that present TLR4/CD14/MD2 receptors – which are efficiently stimulated by LPS, as an important source of pro-inflammatory cytokines, as TNF-α.22 Previous studies of asthmatic children showed an elevated concentration of this cytokine in bronchoalveolar lavage.23 Our data show no difference between severe asthmatic children and healthy controls. This result is probably related with a less differentiation state of blood monocytes and lower expression of TLR. The differentiation into macrophages at lung tissue, in addition to pro-inflammatory environment, increases cell responses and consequently TNF-α production.
Another important pro-inflammatory cytokine is IL-17, which plays an important role in the pathogenesis of asthma, since it is essential in neutrophil recruitment to inflammatory sites. This recruitment could also be observed in bronchial tissue samples,24 and bronchoalveolar lavage and sputum25 from asthmatic patients. In our results, we could evaluate that PBMC from severe asthmatic children produced higher amounts of IL-17 compared to healthy controls. These data are consistent with those found in the literature, showing that asthmatic subjects had higher amounts of IL-17 mRNA when compared with non-asthmatic subjects.26 In addition, severe asthmatic children have higher concentrations of IL-17 in serum than mild and moderate asthmatic children.26
The regulation of this inflammatory process is essential for proper functioning of immune responses and to control exacerbated allergic states. In this context IL-10 acts negatively regulating cytokine production by mononuclear phagocytes, natural killer cells and Th2 lymphocytes.27 We found a higher production of IL-10 by PBMC of asthmatic children. Once IL-10 gene promoter presents common transcription factors to pro-inflammatory cytokines, this cytokine release does not constitute a conflicting result. Still, despite IL-10 production, IL-17 and IFN-γ release was more significant to asthma clinical features.27
In summary, we demonstrate that PBMC from severe asthmatic children produce lower amounts of important cytokines for adequate immune response, such as IL-12. These data corroborate with our recent study in a prospective cohort of recurrent wheezing infants early exposed to high levels of endotoxin. In this study, LPS-stimulated PBMC release significantly less IL-12 and IFN-γ compared to non-recurrent wheezing infants. Our study, with established asthmatic children, supports the hypothesis that asthma and probably other atopic diseases present an inherited hyporegulation of the IFN-γ/IL-12 axis leading to a defective Th1 response, and a consequent predisposition to a relative exacerbation of Th2 response.
Ethical disclosuresProtection of human subjects and animals in researchThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Conflict of interestThe authors have no conflict of interest to declare.
The authors thank Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP – n: 2006/6529834 and 2007/55779-1) for financial support.