INTRODUCTION
Intravenous immunoglobulin (IVIG) is a product administered for several pathologies, with excellent therapeutic response. In patients with primary immunodeficiency (PI), secondary immunodeficiency (SI), autoimmune diseases (AD), systemic inflammatory diseases (SID), allergic diseases, or infections, the pharmacological benefit of IVIG is carried out through different action mechanisms: replacement or substitution therapy 1,2, Fc receptors blocking 3-6, production suppression and neutralization of cytokine 7,8, idiotype-anti-idiotype regulation, among others.
IVIG preparations must comply with all the regulatory requirements established by the World Health Organization (WHO) 9 and the European Pharmacopoeia (EP) 10 for clinical tolerance, therapeutic efficacy, and viral safety.
Although IVIG are efficient and safe products, in some patients they may cause adverse effects such as fever, dyspnea, headache, nausea, vomiting, chills 11-16.
The Laboratorio de Hemoderivados, which belongs to the National University of Córdoba, Argentina, is the largest state-owned, non-profit fractionation plant for plasmatic proteins in South America. It produces medicines from plasma obtained from voluntary non-remunerated healthy donors from Argentina, Uruguay and Chile.
In 1997 the plant began to produce lyophilized Intravenous Immunoglobulin UNC (lyophilized IVIG UNC®) from Southern Cone plasma, which meant a great contribution to the regional public health since up to that moment, the IVIG available were of European or North American origin.
From the epidemiological point of view, the origin of the plasma used for the production of this immunoglobulin means an advantage because it contains specific antibodies to the most prevalent etiological agents existing in this zone of South America. Another advantage is related to socio-economical aspects because as from the moment it entered the market, the product became more accessible for patients.
Since its commercialization, a pharmacovigilance study was initiated 17 with the objective of evaluating the clinical tolerance, therapeutic efficacy, and viral safety of this regional IVIG. For that purpose, a Pharmacovigilance Report was designed in accordance with the regulations of the European Agency for the Evaluation of Medicinal Products (EMEA) 18 and the Latin American Group for Primary Immunodeficiencies (LAGID) 19.
In order to improve the pharmaceutical form, changes were made both to the productive process and to the final formulation. In 2001, it began the production of liquid Intravenous Immunoglobulin UNC (liquid IVIG UNC®) started. One of the improvements involved substituting sucrose, used as stabilizing excipient for the immunoglobulin G molecule, for sorbitol. This modification was made taking into account the characteristics necessary for the stabilizer of the new pharmaceutical form of the product, and also considering the reports published at that time, which suggested that IVIG containing sucrose might pose the risk of acute renal failure 20-22. On the other hand, it should be emphasized that the liquid pharmaceutical form represents an advantage from the drug administration standpoint since it is ready to be used and it does not need to be redissolved, with the potential risk of contamination that this process implies.
A pharmacovigilance follow-up of the new form was conducted using the same tools as for the lyophilized pharmaceutical form.
The aim of this study was to evaluate the clinical tolerance of the two pharmaceutical forms (lyophilized and liquid) of IVIG UNC® produced by the Laboratorio de Hemoderivados, and to compare the data reported in the pharmacovigilance reports of both pharmaceutical forms.
PATIENTS AND METHODS
Patients
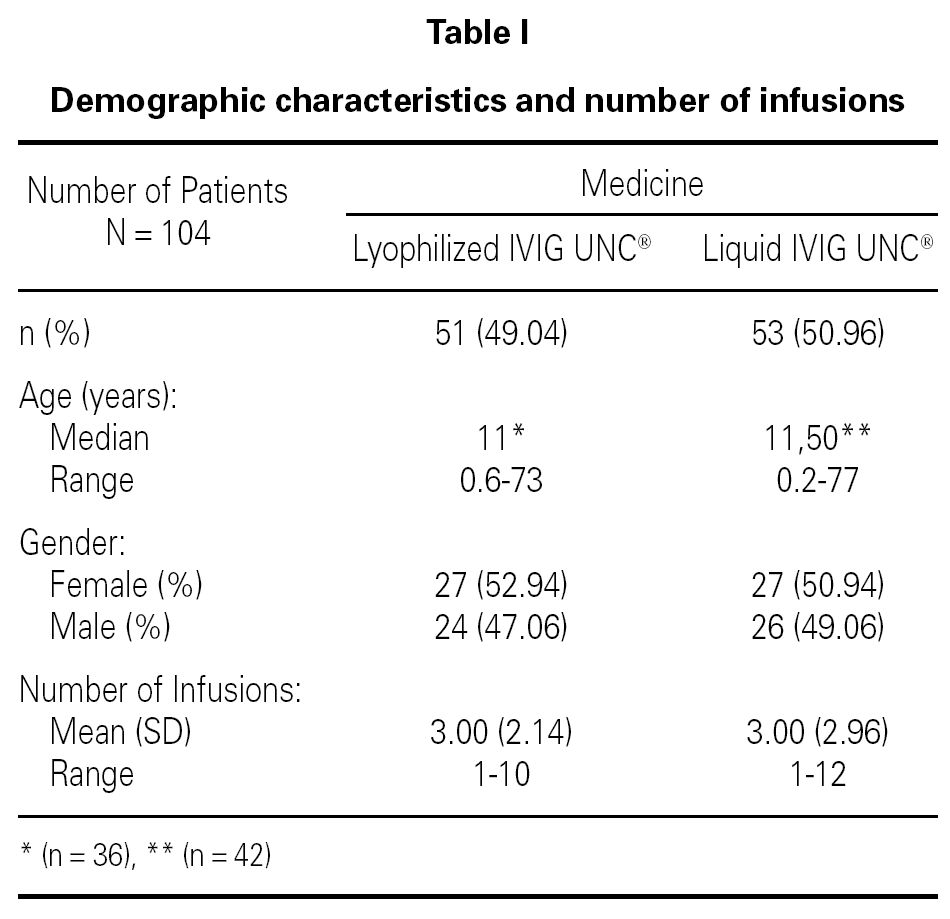
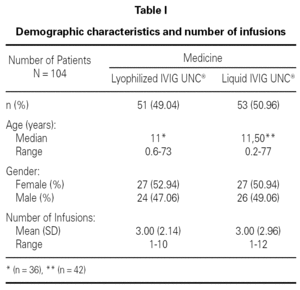
A retrospective study was conducted over the Pharmacovigilance Reports of 149 lyophilized IVIG UNC® infusions administered to 51 patients, and 157 liquid IVIG UNC® infusions administered to 53 patients, all of them ranging from 1 to 77 years old and treated at several health centers of Argentina. Inclusion and exclusion criteria were determined (age, pathology, number of infusions) which allowed the organization of a database with two populations comparable with each other.
The inclusion criteria of the patients for diseases were:
a)Primary Immunodeficiencies (PI)23: agammaglobulinemia, ataxia-telangiectasia, deficit of specific antibodies, hyper IgE syndrome, hypogammaglobulinemia and common variable immunodeficiency, diseases classified as.
b)Autoimmune Disease (AD) 4: autoimmune hemolytic anemia, dermatomiositis, Guillain-Barré Syndrome, Rh incompatibility, systemic lupus erythematosus, myasthenia gravis, pemphigus, and thrombocytopenic purpura.
c)Kawasaki disease, a systemic inflammatory disease (SID) 4.
Medication
Liquid Intravenous Immunoglobulin UNC (ready-to-use sterile solution) and/or its lyophilized form (lyophilized powder to be reconstituted with a solvent at the moment of its use) were used. Both products are produced by the Laboratorio de Hemoderivados of the National University of Córdoba, from a pool of normal human plasma (from voluntary, non-remunerated, healthy donors from Argentina, Uruguay, and Chile), constituted by individual units, serologically nonreactive for: HIV, HBV and HCV, analyzed at the Blood Bank of origin, re-evaluated at the Laboratorio de Hemoderivados, and controlled using polymerase chain reaction technology (PCR) for hepatitis C virus, in accordance with the European Union requirements 24. The origin of the plasma used ensures that this medicine biological origin, in addition to being the only one with regional character, contains antibodies directed towards infectious agents typical of this zone of South America.
IVIG UNC® 25 is elaborated from Cohn Fraction II (F II) by treatment at acid pH with traces of pepsin 26, obtaining a product where the IgG maintains its structure as much as its biological activity intact. The manufacturing process includes three steps for viral inactivation: cold-ethanol fractionation, pasteurization, and enzymatic treatment at acid pH, which makes this product highly safe 27.
Data Collection
Data were recorded by the physician in a Pharmacovigilance Report designed in accordance with EMEA 18 and LAGID 19 regulations.
This form recommends that the following data be recorded: institution where the infusion was administered; prescribing physician; patient; medication; report on adverse reactions observed; result of the serological analysis of viral markers for hepatitis B and C, and for human immunodeficiency, pre- and post-infusion; clinical evolution; data on specific or general parameters related to the base disease; signature and seal of the professional.
In the Pharmacovigilance Reports as well as in this study, the patients' identities have been kept confidential.
Data Processing
Data recorded by the professionals in the Pharmacovigilance Reports were entered in a database.
The content of such database was checked at random and at regular intervals for data accuracy and comprehension.
Clinical Tolerance
Following other authors' description 19,28,29, adverse reactions were classified into:
Mild: backache, blush, fever, pruritus, erythema, chills, cephalea, nausea, myalgia, anxiety and irritability, generally associated to the rate of infusion. Symptoms disappear when the rate is reduced, and they rarely require interrupting the administration of the infusion.
Moderate: bronchiospasm, panting, vomiting, chest pain, urticaria, tachycardia, or mild progressive symptoms that do not respond to the reduction in the rate of infusion and require interrupting the infusion and initiating an emergency treatment.
Severe: throat tightness, dyspnea, faint and hypotension. Symptoms and signs start seconds or minutes after beginning with the infusion. Other severe reactions, generally associated to the administration of high doses for immunomodulation, include aseptic meningitis, thromboembolic accidents, renal failure, and erythema multiform.
Taking into account the preceding classification, the IVIG UNC® clinical tolerance was established as:
* Excellent tolerance: infusions involving no adverse reactions.
* Very good tolerance: infusions involving mild adverse reactions.
* Regular Tolerance: infusions involving moderate adverse reactions.
* Intolerance: infusions involving severe adverse reactions.
Statistical analysis:
A descriptive analysis of the demographic characteristics of both groups was made, also including the number of infusions, by calculating averages, standard deviations (SD), median and range. Frequency tables were prepared with data on pathology by medicine, and adverse effects, grouped by pathology. The association between these variables was statistically evaluated by Fisher's exact test. Clinical tolerance was evaluated considering four possible response categories: The statistical analysis was carried out using the Pearson test for association between variables.
The following programs were used for the statistical analysis: Epi Info version 6, Statistix 7 and InfoStat v 2005p1.
RESULTS
Patients Description
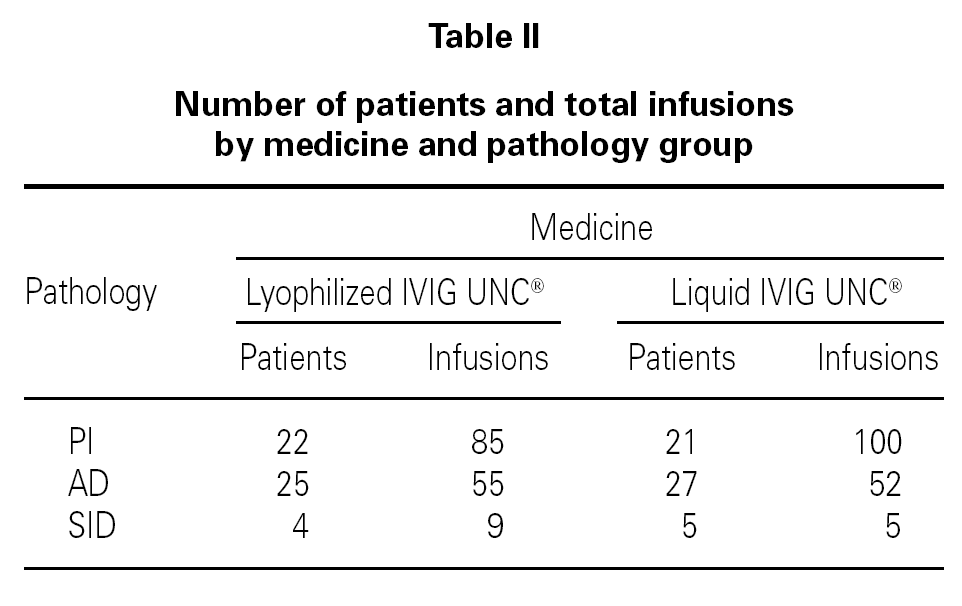
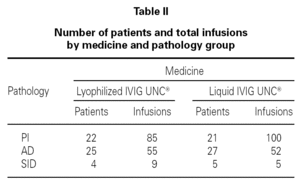
Table I shows the demographical data corresponding to the patients and the number of infusions in each one of the medication pharmaceutical forms. Table II shows the distribution of patients and the total number of infusions administered according to medicine and pathology group.
Clinical Tolerance
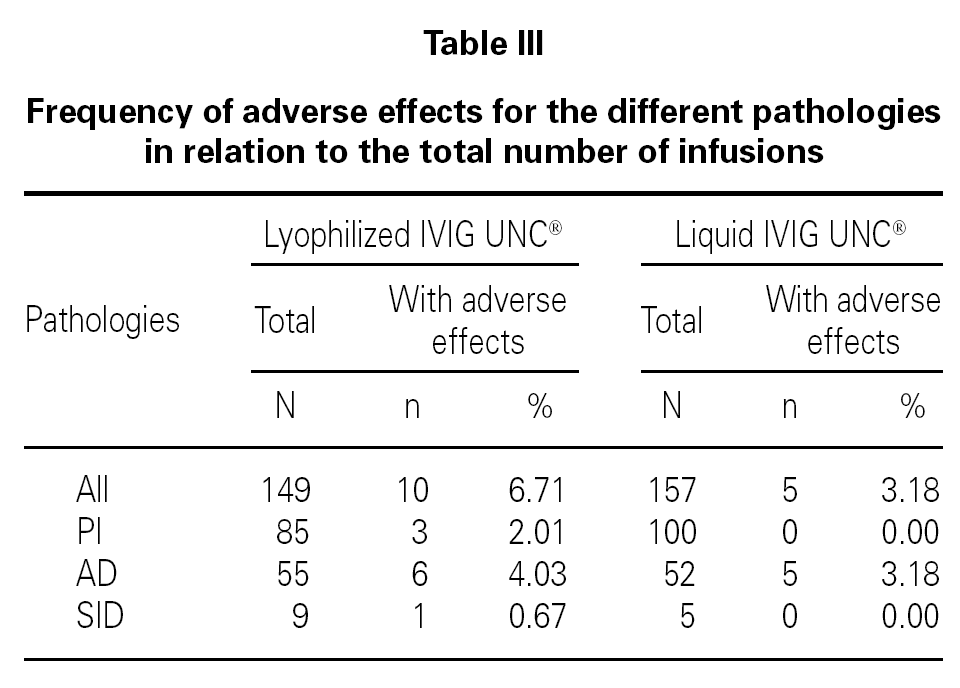
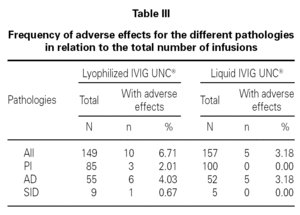
The frequency of adverse reactions for the different pathology groups was analyzed with respect to the total number of infusions (table III).
The statistical analysis shows homogeneity in the proportion of adverse effects for the two populations sampled with relation to the number of infusions administered (p > 0.05).
This analysis also indicates that there is no association between the pharmaceutical form administered and the presence of adverse effects (p > 0.05) for patients with PI, AD, and SID.
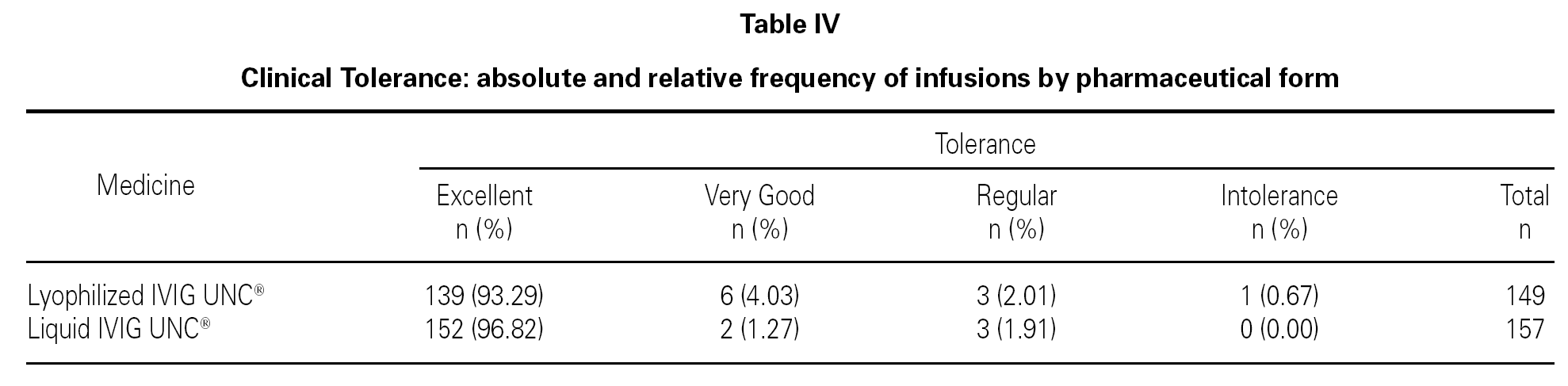
Based on the previously defined parameters, and with the statistical analysis of the data obtained from the reports in relation to the total number of lyophilized and liquid IVIG UNC®infusions, the results observed are shown in table IV.
Analyzing the clinical tolerance response of both pharmaceutical forms, it is possible to observe that there is a greater percentage of infusions with excellent results associated to the liquid pharmaceutical form with respect to the lyophilized form, and a smaller percentage for the remaining options.
However, the statistical analysis did not show association between tolerance and the pharmaceutical form used (p > 0.05), and it indicated a similar tolerance for both preparations.
The probability of demonstrating an adverse effect in patients dealt with lyofilized IVIG UNC® is 0.0671, and 0,0318 for patients dealt with liquid IVIG UNC®. Therefore, the relative risk to suffer an adverse effect from lyofilized IVIG UNC® with respect to liquid IVIG UNC® is twice greater 2,11. Nevertheless the confidence interval determines that this value can be between 0.70 and 5.75 with a 95 % of confidence, indicating independence in the answers for both populations of patients.
In all records analyzed it was reported that IVIG UNC® was therapeutically effective, which agreed with the data obtained from other clinical trials 30-33.
In relation to the viral safety of the product, seroconversions generated by the administration of lyophilized or liquid IVIG UNC® were not reported.
DISCUSSION
Administration of IVIG for several pathologies produces an excellent therapeutic response, carried out through different action mechanisms.
In some patients, the IVIG infusion may cause the onset of adverse reactions 34-36 due to the presence in the product of IgG aggregates that activate the complement system, or due to the presence of contaminants or even stabilizers used during the production process. Generally, these signs disappear when the rate of administration is reduced. However and less frequently, these symptoms remain for some days, possibly due to allergic complications.
Most adverse reactions could be reduced taking into account aspects such as the use of purification methods that ensure the quality of the product, compliance with the requirements established by the World Health Organization (WHO) 9 and the European Pharmacopoeia (EP) 10, the study of factors that cause the patient to be predisposed to experience adverse effects, and compliance with the instructions to administer the medicine.
On the basis of the first two premises, from 1997 a regional IVIG began to be produced. It was manufactured by treatment at acid pH with traces of pepsin, a procedure that eliminates IgG aggregates without altering the physiological properties of monomers. The resulting product meets all the quality requirements established by national and international regulatory agencies.
The production of IVIG UNC® started with the lyophilized pharmaceutical form. Later on and with the aim to improve the product and make it easier to administer, changes were made to the production process and to the final formulation. The result was the liquid IVIG UNC®, that started to be commercialized in 2001.
Since the production of lyophilized IVIG UNC® started, a pharmacovigilance study began in order to evaluate its therapeutic efficacy, clinical tolerance, and viral safety. The data obtained were recorded in a Pharmacovigilance Report designed in accordance with the European Agency for the Evaluation of Medicinal Products (EMEA) 18 and of the Latin American Group for Primary Immunodeficiencies (LAGID) 19 regulations. Based on the information collected and analyzed, a retrospective study 17 was conducted. The conclusion was that the medicine was effective, well tolerated, and safe.
Since liquid IVIG UNC® was first produced, a pharmacovigilance follow-up of the new form was conducted following the same criteria used for the lyophilized form.
In both pharmaceutical forms, medical professionals were very strict when informing the adverse reactions. However, neither lack of therapeutic efficacy nor seroconversions after administration of IVIG UNC® were reported.
Considering these matters, the aim of this study was to evaluate the clinical tolerance of the two pharmaceutical forms (lyophilized and liquid) of IVIG UNC®, and to compare the adverse reactions reported in the pharmacovigilance reports. All data were kept in a general database and, applying inclusion and exclusion criteria, two comparable populations extracted from that database were determined.
One hundred and forty-nine infusions administered to 51 patients and 157 infusions administered to 53 patients for the lyophilized and liquid forms respectively were analyzed. From the total, 93.3 % were reported without adverse reactions in lyophilized IVIG UNC® and 96.8 % in liquid IVIG UNC®. The liquid form showed a greater percentage of infusions with excellent tolerance. Despite the difference, these results were statistically not significant.
The frequency of total adverse reactions for the lyophilized IVIG UNC® was 6.7 %, from which 4.0 % was mild, and for the liquid form of IVIG UNC® was 3.2 %, from which 1.3 % was mild, in relation to the total of infusions recorded for each product.
Neither anaphylactic nor hemodynamic reactions as a consequence of the use of these products were reported. Only one mild meningeal case which occurred 4 days after a patient was treated with lyophilized IVIG UNC® was reported, but it was not attributable to the product and it evolved favorably without leaving sequels. Other authors described this type of signs when other IVIG commercial brands were used 14-16.
The incidence of the adverse effects reported for both pharmaceutical forms of IVIG UNC® is below the incidence mentioned by Stiehm 29, who reported that mild adverse effects associated to the administration of IVIG are common and occur in approximately 10 % of the infusions. According to the results obtained in this study, we can affirm that IVIG UNC®, in its two pharmaceutical forms, is a safe product with an excellent clinical tolerance.
Correspondence:
A.C. Mahieu
Laboratorio de Hemoderivados
Universidad Nacional de Córdoba
Avda. Valparaíso, s/n. Ciudad Universitaria.
X5000HRA Córdoba. Argentina
Tel.: 54 351 4334122/23
Fax: 54 351 4334124
E-mail: caromah@hemo.unc.edu.ar