Polymorphisms of plasminogen activator inhibitor-1 (PAI-1) and angiotensin-converting enzyme (ACE) genes have been implicated in susceptibility to asthma. In this study, we aimed to investigate whether there was any association between childhood asthma and polymorphisms of the PAI-1 and ACE genes.
MethodsTwo hundred and three Turkish children aged 5–15 years, including 102 asthmatic patients and 101 healthy control subjects were included in this study. The asthma group was divided into two groups as follows: Group I: Asthmatic children with positive family history for atopy (n=53), Group II: Asthmatic children without any family history for atopy (n=49). One hundred and twenty-eight atopic family members were also included in the study. The insertion/deletion (I/D) polymorphism of the ACE and PAI-1 4G/5G gene polymorphisms was carried out by polymerase chain reaction.
ResultsThe prevalence of the PAI-1 4G allele was significantly greater in asthmatic children compared to control group (p<0.05, OR: 1.64 (1.11–2.43)) but there was no significant relation between ACE I/D genotypes and childhood asthma. No significant difference was detected between Groups I and II in terms of these ACE and PAI-1 genotypes and allele frequencies. No significant relationship was found between both gene polymorphisms and total serum IgE and skin prick test results.
ConclusionIt has been established that PAI-1 4G allele may be a genetic risk factor for childhood asthma but ACE gene I/D polymorphisms do not play a role in the development of asthma in the sample of Turkish children.
Asthma is an obstructive pulmonary disease characterized by bronchial hyperresponsiveness, airway inflammation, epithelial injury and airway smooth muscle hypertrophy.1 It is a common multifactorial disease which is influenced by both genetic and environmental factors.
Several genes are identified as asthma susceptible genes, one of which is the plasminogen activator inhibitor-1 (PAI-1) gene. PAI-1 is a glycoprotein that belongs to the serine protease inhibitor superfamily and has a primary function in the regulation of fibrinolytic enzyme system. Disturbance of the balance between proteases and inhibitors is an important predictor of the pathological processes of tissue repair and remodelling.2 PAI-1 is closely associated with fibrosis and accumulation of extracellular matrix after lung injury or inflammation and it has an essential role in airway remodelling of asthma.3,4 The PAI-1 gene has been localized to chromosome 7 and deletion/insertion polymorphisms (4G/5G) have been found to be correlated with plasma levels of PAI-1.5 Inflammatory stimuli like interleukin-1 (IL-1) and tumour necrosis factor (TNF)-α increase plasma levels of PAI-1 and it has been shown that the 4G allele produces six times more RNA compared to 5G allele in response to inflammatory stimuli.5 Basal plasma PAI-1 levels were also found to be higher in individuals homozygous for the 4G allele.5 There are several reports indicating that 4G allele of the 4G/5G polymorphism in the PAI-1 gene is associated with IgE-mediated asthma and allergic diseases.6–9 Because gene-environment interactions differ between populations, ethnic differences in the polymorphisms of PAI-1 and association between the disease may be present. There may also be different results among the same ethnic populations so new studies with larger patients are needed.10,11
Another candidate gene proposed to be involved in the pathogenesis of asthma is angiotensin converting enzyme (ACE) gene. Angiotensin converting enzyme plays an essential role in the metabolism of angiotensin II (AT II) and inactivates bradykinins and tachykinins which are bronchoconstrictors and also mediators of inflammation in asthma.12 Angiotensin II is also involved in the pathogenesis of asthma by interaction with airway smooth muscle. An insertion/deletion polymorphism (I/D) of the ACE gene in intron 16 has been identified and shown to correlate with plasma ACE levels.13 A genetic association has been investigated between the asthma and I/D polymorphism of ACE gene in many studies14–21 but results are still inconclusive.
Recent literature about Turkish asthmatic populations revealed conflicting results concerning the associations between PAI-1 4G/5G and ACE I/D polymorphisms and asthma.10,11,16,21 In this study we aimed to evaluate the possible association between these PAI-1 and ACE polymorphisms and asthma in Turkish children.
Material and methodsCharacteristics of the patientsTwo hundred and three Turkish children aged 5–15 years, including 102 asthmatic patients and 101 healthy control subjects, participated in this study. Asthmatic children were recruited from Dokuz Eylul University, Pediatric Allergy Department. Asthma was diagnosed by a history of intermittent wheezing and the presence of reversible airway obstruction as defined by at least a 12% improvement in final expiratory volume in 1s (FEV1) following bronchodilator administration; and asthma severity grading was made according to Global Initiative for Asthma (GINA) guidelines.22 Briefly, the severity of a patient's asthma was classified into mild, moderate or severe category based on the clinical features present before treatment was begun. When the patient was already on treatment, the classification of severity was based on the clinical features present and the step of the daily medication regimen that the patient was currently on. Thus, a patient with ongoing symptoms of mild persistent asthma, despite being on the appropriate maintenance treatment for this step, was regarded as having moderate persistent asthma. Subjects with mild and moderate asthma were included in the study and severe asthmatics were excluded. The control group consisted of patients from the Pediatric Outpatient Clinic of the same university who denied any respiratory symptoms and had no personal and family history of asthma or allergy. The asthma group was divided into two groups as follows: Group I: Asthmatic children with positive family history for atopy (n=53); and Group II: Asthmatic children without any family history for atopy (n=49). One hundred and twenty-eight atopic family members were also included in the study. Asthmatic patients with any other chronic diseases and those who had experienced an acute asthma attack within the previous month were excluded from the study.
Serum total IgE levels, skin prick tests (SPTs) and genotyping of ACE and PAI-1 genes were performed in the asthmatic group while only genotyping of ACE and PAI-1 genes were performed in the control group and atopic family members of the asthmatic patients.
This study was approved by the ethics committee of Dokuz Eylul University Faculty of Medicine. All participants provided written informed consent.
Skin prick tests and total serum IgEAsthmatic subjects underwent SPTs with a panel of aeroallergens (Allergopharma, Reinbek, Germany) including house dust mites (Dermatophagoides farinae and Dermatophagoides pteronyssinus), weed mix (Artemisia vulgaris, Urtica dioica, Tarax acum vulgare, Plantago lanceolata), grass and cereal mix (Holcus lantus, Dactylis glomerata, Lolium perenne, Phleum pretense, Poa pratensis, Festuca pratensis, Hordeum vulgare, Avena sativa, Secale cereale, Triticum sativum), tree mix (Alnus glutinosa, Corylus avellana, Populus alba, Ulmus scabra, Salix caprea, Betula verrucosa, Fagus silvitica, Quercus robur, Platanus orientalis), mould mix (Alternaria alternata, Botrytis cinerea, Cladosporium herbarum, Curvularia lunata, Fusarium moniliforme, Helminthosporium halodes, Aspergillus fumigatus, Mucor mucedo, Penicillium notatum, Pullularia pullulans, Rhizopus nigricans, Serpula lacrymans), animal dander mix (Golden hamster, dog, cat, rabbit, guinea pig epithelia) and olea and pine tree. Histamine hydrochloride of 10mg/mL and NaCl of 9mg/mL were used as positive and negative controls, respectively. A mean wheal diameter of 3mm was considered as positive.
Total serum IgE was considered as ‘high’ if it was higher than 50IU/mL according to our laboratory ranges.
Genetic analysisAssay for identification of plasminogen activator inhibitor-1 and angiotensin converting enzyme gene polymorphisms is based on polymerase chain reaction (PCR) and reverse-hybridization with CVD Strip Assay (ViennaLab Labordiagnostica GmbH, Austria). The assay included three steps: Genomic DNA was isolated from peripheral blood leukocytes with use of proteinase K/SDS digestion, followed by “salting out” extraction, according to standard procedures.23 DNA samples were amplified biotinylated in multiplex PCR reactions for 35 cycles of denaturation at 94°C for 15s, annealing at 58°C for 30s, and extension at 72°C for 30s, followed by a final extension step at 72°C for 3min, and hybridized for 30min at 45°C to a membrane test strip. Finally, the amplification products are selectively hybridized to a test strip, which contains allele-specific (wild type and mutant) oligonucleotide probes immobilized as an array of parallel lines. Bound biotinylated sequences are detected using streptavidin-alkaline phosphatase and colour substrates.24
StatisticsData were analysed with SPSS software (Scientific Package for Social Sciences, Version 15.0, SPSS, Inc., Chicago, IL, USA). The χ2-test was performed to test for comparison of differences in genotype and SPTs. Serum IgE levels among the groups were analysed with Mann–Whitney test. Odds ratio and 95% confidence interval values were calculated for demonstration of association between asthma and PAI-1 4G/5G and ACE I/D polymorphisms. A value of p=0.05 or less was considered to be significant.
ResultsThe characteristics of asthmatics and the control group are shown in Table 1. There were no differences between Groups I (asthmatic children with positive family history for atopy) and II (asthmatic children without any positive family history for atopy) by means of age and gender. Skin prick test was positive in 29 patients (55%) of Group I while it was also positive in 29 patients (59%) of Group II. Total serum IgE levels were higher in Group I (median total IgE: 186IU/mL) than Group II (median total IgE: 98.1IU/mL) (p=0.01).
The PAI-1 4G/5G genotype distributions and allele frequencies in the study groups are shown in Table 2. The prevalence of the 4G allele was higher in asthma group while 5G allele was higher in controls (p<0.05, OR:1.64, CI: 1.11–2.43). The frequencies of genotypes were also significantly different in asthmatics and controls, 4G/5G being higher in asthmatics (p<0.05, OR: 2.17, CI: 1.24–3.81). There was no significant difference between Groups I and II in terms of PAI-1 alleles and genotypes (p>0.05). Characteristics of the patients such as SPT results and IgE levels according to PAI 4G/5G genotypes and alleles are presented in Table 3.
PAI-1 4G/5G genotype distributions and allele frequencies in study group.
| Asthmatics(n=102)n (%) | Control(n=101)n (%) | Group I(n=53)n (%) | Group II(n=49)n (%) | |
| 4G | 111(54) | 85(42) | 56(53) | 55(56) |
| 5G | 93(46) | 117(58) | 50(47) | 43(44) |
| p | <0.05a | >0.05b | ||
| OR (95% CI) | 1.64 (1.11–2.43)a | 0.87 (0.5–1.52)b | ||
| 4G/4G | 24(23) | 21(21) | 12(23) | 12(25) |
| 4G/5G | 63(62) | 43(42) | 32(60) | 31(63) |
| 5G/5G | 15(15) | 37(37) | 9(17) | 6(12) |
| p | <0.05a | >0.05b | ||
| OR (95% CI) | 2.17 (1.24–3.81)c | 0.88 (0.39–1.97)c | ||
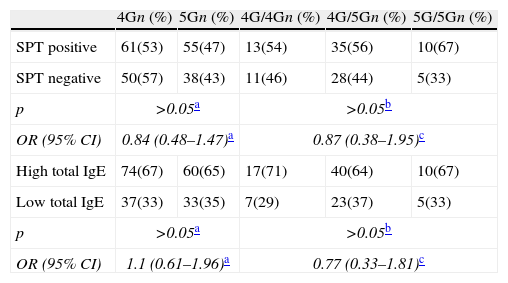
Characteristics of the patients according to the PAI-1 4G/5G genotype and alleles.
| 4Gn (%) | 5Gn (%) | 4G/4Gn (%) | 4G/5Gn (%) | 5G/5Gn (%) | |
| SPT positive | 61(53) | 55(47) | 13(54) | 35(56) | 10(67) |
| SPT negative | 50(57) | 38(43) | 11(46) | 28(44) | 5(33) |
| p | >0.05a | >0.05b | |||
| OR (95% CI) | 0.84 (0.48–1.47)a | 0.87 (0.38–1.95)c | |||
| High total IgE | 74(67) | 60(65) | 17(71) | 40(64) | 10(67) |
| Low total IgE | 37(33) | 33(35) | 7(29) | 23(37) | 5(33) |
| p | >0.05a | >0.05b | |||
| OR (95% CI) | 1.1 (0.61–1.96)a | 0.77 (0.33–1.81)c | |||
The frequencies of the PAI-1 gene alleles and polymorphisms in 128 atopic family members of asthmatic group were as follows: 105 (82%) with 4G allele, 23 (18%) with 5G allele, 30 (23.4%) with 4G/4G, 75 (58.6%) with 4G/5G and 23 (18%) with 5G/5G polymorphisms. The 4G allele was more common than the 5G allele in atopic family members.
The ACE I/D genotype distributions and allele frequencies in the study groups are shown in Table 4. There were no significant differences between controls and asthmatics. Also, there was no significant difference between Groups I and II in terms of these ACE genotypes and allele frequencies.
ACE I/D genotype distributions and allele frequencies in study group.
| Asthmatics(n=102)n (%) | Control(n=101)n (%) | Group I(n=53)n (%) | Group II(n=49)n (%) | |
| D | 120(59) | 104(52) | 58(55) | 62(63) |
| I | 84(41) | 98(48) | 48(45) | 36(37) |
| p | >0.05a | >0.05b | ||
| OR (95% CI) | 1.34 (0.9–1.99)a | 0.7 (0.4–1.2)b | ||
| DD | 32(31) | 22(22) | 15(28) | 17(35) |
| ID | 56(55) | 60(59) | 28(53) | 28(57) |
| II | 14(14) | 19(19) | 10(19) | 4(8) |
| p | >0.05a | >0.05b | ||
| OR (95% CI) | 1.64 (0.89–3.08)c | 1.34 (0.58–3.11)c | ||
The frequencies of the ACE gene I/D alleles and polymorphisms in 128 atopic family members of asthmatic group were as follows: 98 (77%) with D allele, 30 (23%) with I allele, 41 (32%) with DD, 57 (45%) with ID and 30 (23%) with II polymorphisms. The D allele and ID genotype of ACE gene were more common in atopic family members.
Characteristics of the patients such as SPT results and IgE levels according to ACE I/D genotypes and alleles are presented in Table 5.
Characteristics of the patients according to the ACE I/D genotype and alleles.
| Dn (%) | In (%) | DDn (%) | IDn (%) | IIn (%) | |
| SPT positive | 64(53) | 52(62) | 16(50) | 32(57) | 10(71) |
| SPT negative | 56(47) | 32(38) | 16(50) | 24(43) | 4(29) |
| p | >0.05a | >0.05b | |||
| OR (95% CI) | 1.42 (0.8–2.5)a | 0.66 (0.28–1.54)c | |||
| High total IgE | 76(63) | 58(69) | 21(66) | 34(61) | 12(86) |
| Low total IgE | 44(37) | 26(31) | 11(34) | 22(39) | 2(14) |
| p | >0.05a | >0.05b | |||
| OR (95% CI) | 1.29 (0.71–2.33)a | 0.99 (0.41–2.4)c | |||
Asthma and allergic diseases are polygenic disorders and also represent genetic heterogeneity. PAI-1 and ACE genes are potential candidate genes supposed to be involved in the pathogenesis of asthma. In this study we analyzed the distribution of PAI-1 gene 4G/5G and ACE gene I/D polymorphisms in Turkish asthmatic children and their atopic family members and tried to determine if atopy and total serum IgE levels correlated with specific genotypes or not.
This present study indicates that asthmatic children and their atopic family members had a higher frequency of 4G allele of the PAI-1 gene. Our findings are mainly consistent with the previous literature. There are adult studies from different ethnic groups indicating that PAI-1 4G allele is more common in asthmatic patients compared with controls.6–8 Two studies from Poland are mainly based on allergic asthma patients and they conclude that PAI-1 4G allele is related with both atopy and asthma.6,8 Total IgE levels in individuals with 4G alleles were also significantly higher than 5G/5G homozygotes in these studies. We did not find any association between positive SPTs and total serum IgE levels in our study. This may be explained by ethnic differences regarding genetic determination of IgE levels. Another report based on data of asthmatic children and their family members also concluded that the prevalence of 4G allele was higher in asthma population.7 This study reported that 4G allele was preferentially transmitted to asthma-effected children but atopy was not related with 4G allele. We found a similar distribution in atopic and non-atopic asthmatic subgroups but the small number of patients in these subgroups is a limitation of our study and this may be the reason for this result. Our study is the second study investigating genotypes of family members of asthmatic children. We investigated only atopic family members and found that 4G allele was more common in this group but we cannot make any further conclusions about 4G allele transmission to atopic and non-atopic asthmatic children.
Two studies from Turkey report conflicting results about the relation of PAI-1 4G/5G gene polymorphisms and asthma.10,11 Cosan et al.10 suggested that 4G/5G polymorphisms of PAI-1 gene do not play a role in the development of asthma in the Turkish population. This adult study also stated that there were no differences between atopic and non-atopic asthmatics with respect to genotype distribution. Ozbek et al.11 studied children population with asthma and allergic rhinitis and found that Turkish children with asthma or allergic rhinitis have a higher prevalence of PAI-1 4G allele compared with healthy peers. This study also found no relationship between PAI-1 4G/5G polymorphisms and total serum IgE levels, total eosinophil count and SPT results. Our study and the mentioned study support the hypothesis that PAI-1 gene 4G/5G polymorphism may be a risk factor for childhood asthma in the Turkish population.
The insertion–deletion (I/D) polymorphisms of ACE gene have also been implicated in susceptibility to asthma as it affects serum ACE levels, but many studies carried out before reported inconclusive results. Angiotensin converting enzyme DD genotype has been reported as more prevalent in asthmatics.15,17 Gao et al.17 also stated that ACE DD genotype is related with more severe presentation. But Chagani et al.20 found no association between ACE I/D genotypes and asthma or asthma severity. Similar results were also reported by Lee et al.18 and Nakahama et al.25 A recent meta-analysis suggested that I/D polymorphism of the ACE gene would be a risk factor for asthma.26 Results of this meta-analysis indicated that DD homozygote carriers had almost 59% increased risk of asthma and this risk is more evident in Asians, and this polymorphism was significantly associated with increased asthma risk in children. Five children studies were included in this meta-analysis and six different ethnic groups were studied. Studies from China stated that DD genotype was associated with childhood asthma and Lue et al.27 concluded that DD genotype might play a role in the development of asthma phenotype in children with allergic rhinitis.28,29 Other studies including children from UK/Ireland, South Africa and Japan stated that there was not any relation between ACE I/D genotypes and childhood asthma.13,17 We also found a similar distribution of ACE DD genotype in Turkish asthmatic children and the control group. There was also no association between atopy, serum IgE levels and ACE I/D genotypes in our study, as was the case with the Japanese study by Gao et al.19
There is no study which reported the relationship between ACE I/D genotype and childhood asthma from our country. Adult studies also show different results. Yildiz et al.21 and Urhan et al.30 conclude that ACE gene I/D polymorphisms are not an important determinant of asthma susceptibility in the adult Turkish population but Eryuksel et al.16 suggest that ACE DD gene polymorphism may be a risk factor for asthma.
In summary, we found an association between PAI-1 gene polymorphism and asthma susceptibility in Turkish asthmatic children but we did not find a similar result about ACE I/D polymorphisms. Different results from the literature about PAI-1 4G/5G and ACE I/D polymorphisms may be explained by the genetic heterogeneity and multifactorial etiology of asthma. Multicentre studies with a large number of patients are required to confirm these results.
Conflict of interestThe authors have no conflict of interest to declare.